One of the 4 components of a lithium-ion battery, the cathode is a key that determines the competitiveness of the battery. Since a cathode is made by combining different raw materials, it comes in a variety of combinations with different structures. We will look into the classifications of cathode structures through the infographics.
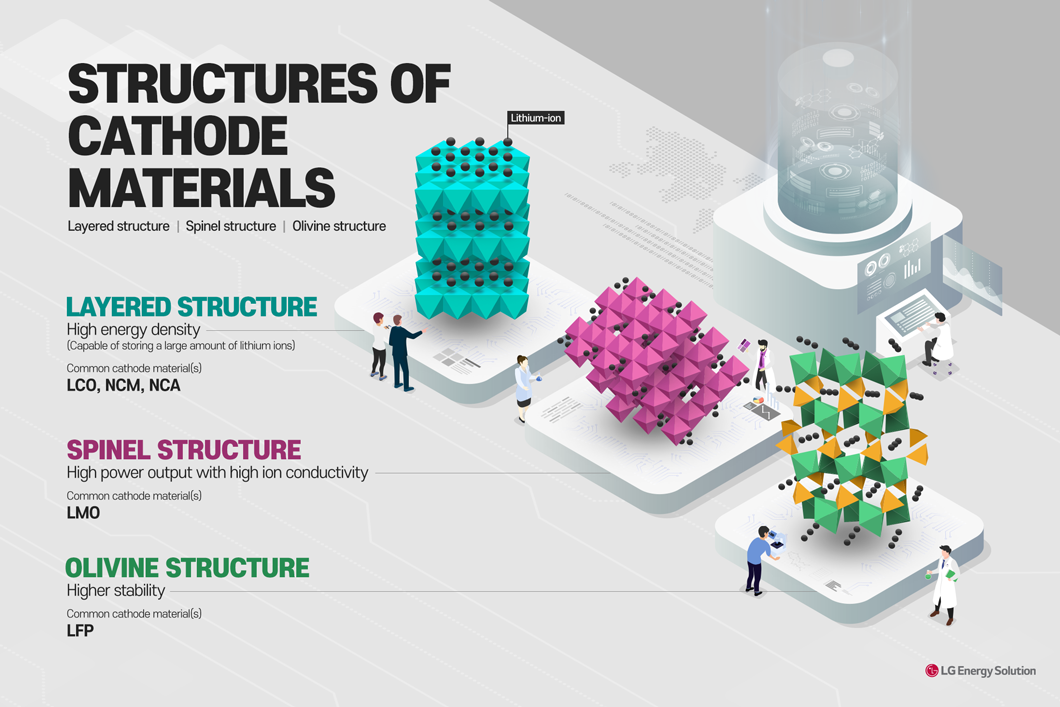
Cathode active materials that make up the cathode are typically composed of lithium, oxygen, and other metal elements. Cathodes can be divided into layered, spinel, and olivine structures.
First, in the layered structure, octahedral layers made of oxygen are stacked up in a regular pattern. There are large and flat spaces between the layers, enough to store a lot of lithium-ions. Also, the two-dimensional channels of the structure allow a high diffusion speed of lithium ions. Common cathode materials with a layered structure include lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (LNCMO or NCM), and lithium nickel cobalt aluminum oxide (LNCAO or NCA).
The spinel structure is common in oxides. One of the widely known cathodes with this structure is lithium manganese oxide (LMO). While the layered structure has two-dimensional channels, the spinel structure has three-dimensional channels because it usually forms a lattice of tetrahedrons and octahedrons. Such features provide the spinel structure with high stability, high output, and a variety of channels through which lithium ions are inserted.
Last, the olivine structure has a hexagonal shape. It has high stability since the strong P-O (phosphate – oxygen) bond allows it to maintain the structure even if all active lithium ions are diffused. Lithium iron phosphate (LFP) is one of the cathodes with this structure.
We learned that there are a variety of cathode materials and that they have different features in their structure through the infographics. We will continue walking you through battery information next time!