There are crucial materials for producing electrical energy in a battery. You need to know about them if you want to understand the structure of lithium-ion batteries and battery manufacturing processes. So, we will delve into what active materials are and what they do.

What is Active Material?
Active materials produce electrical energy through chemical reactions at the cathode and anode. The active material in the cathode is called the “cathode active material,” and the active material in the anode is called the “anode active material.”
Cathode Active Materials
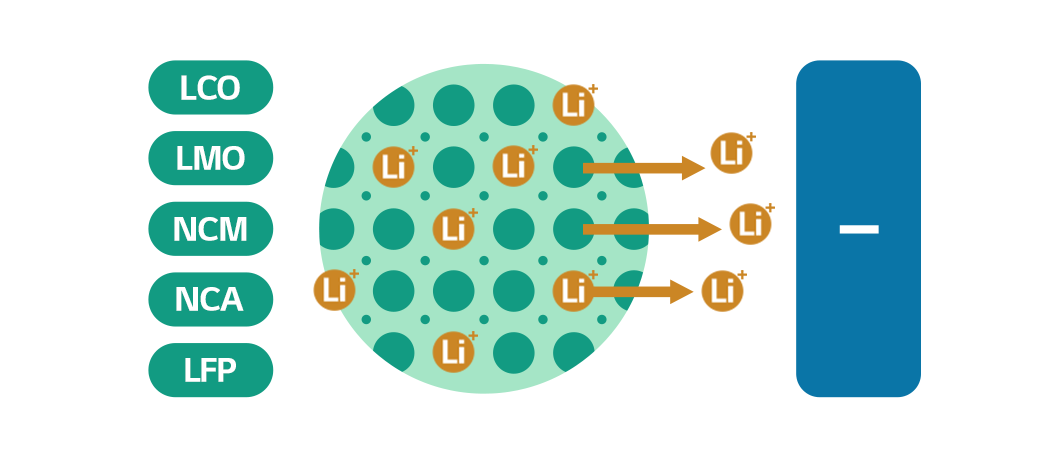
The cathode active materials in the cathode hold lithium ions and send them to the anode as a battery charge. Cathode active materials are responsible for battery capacity and output. To make one, different materials are combined considering their properties and need, resulting in LCO (Lithium cobalt oxide), LMO (Lithium manganese oxide), NCM (Lithium nickel cobalt manganese oxide), NCA (Lithium nickel cobalt aluminum oxides), and LFP (Lithium iron phosphate).
Anode Active Materials
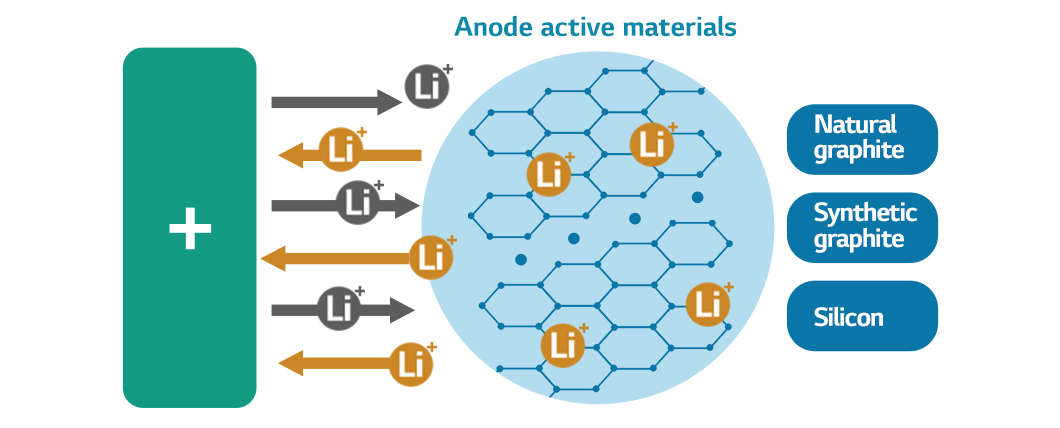
When batteries discharge, the anode active materials in the anode store the lithium ions delivered from the cathode and release them to produce energy. Graphite is the most widely used ingredient for anode active materials since it offers stability when lithium ions travel (charging and discharging). These days, energy-dense silicon is increasingly replacing graphite to improve the performance of the anode.

Since ingredients for active materials are critical in manufacturing batteries, a stable supply of them is important. LG Energy Solution has not only secured the materials, but also is practicing ESG through its responsible supply chain management.





