It is one of the most important challenges to improve the battery life and performance when it comes to the development of batteries. In the effort to achieve the goal, the term “SEI” comes up as a key factor. What is SEI and how does it affect the battery life and the performance?

What is SEI?
The “SEI” refers to the thin layer formed on the surface of the anode during the initial battery charging. When a battery is charged for the first time, the lithium ions in it move to the anode prompting the initial decomposition of electrolyte. This chemical reaction creates a solid layer called SEI, or Solid Electrolyte Interphase, on the surface of the anode.
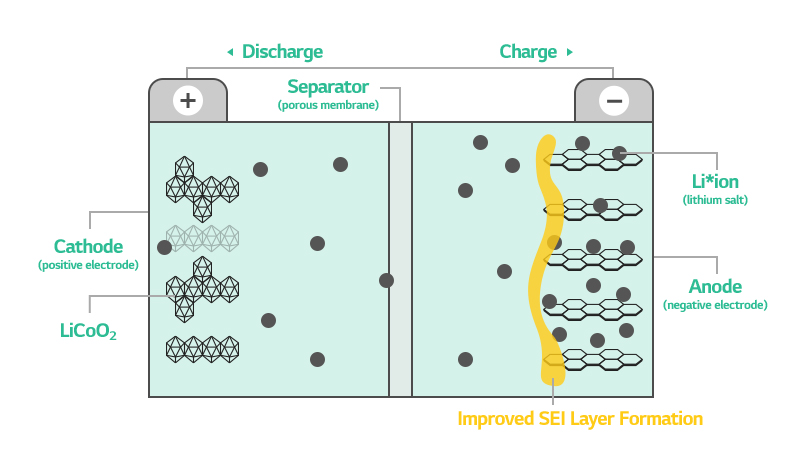
How Batteries Work
To know what the SEI does in a battery, you should first understand how batteries work. Batteries generate power when lithium ions move between the cathode and anode. During charging, lithium ions and electrons both travel from the cathode to anode but in different routes: electrons through the external circuit and lithium ions electrolyte.
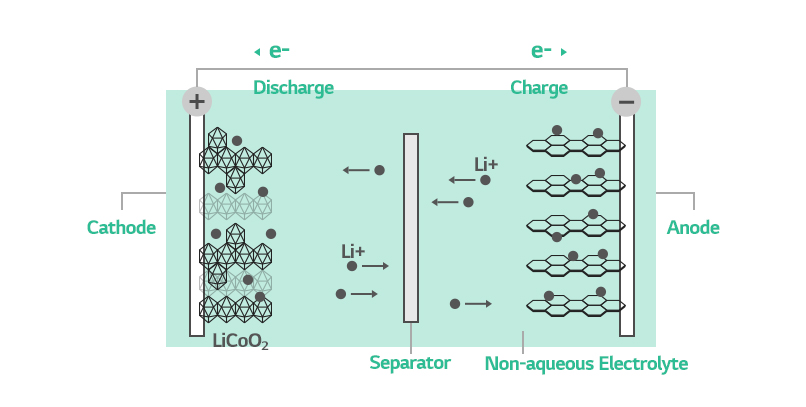
The Role of the SEI
The SEI prevents further decomposition of electrolyte, the path of lithium ions, while allowing transportation of only lithium ions. In other words, it protects electrolyte and works like another separator at the same time.
The most important part in SEI creation is to form the right film. Otherwise, the SEI cannot fulfill its due roles as a separator and a transporter of lithium ions, eventually degrading the battery life and performance.
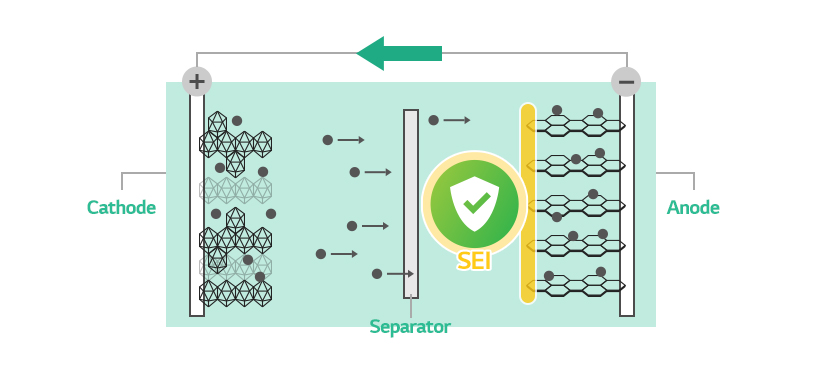
Many of you may not have been familiar with the term SEI. But as you can see, it is crucial in deciding battery life and performance. You will now find some news and data about the batteries easier to understand with the knowledge of SEI. Remember that the SEI is playing a small but essential role whenever you try to charge your electronic devices!