Silicon can determine the charging speed of electric vehicles.
The anode that contains silicon instead of graphite, a widely used anode material for rechargeable batteries, can quadruple a battery’s capacity per gram. Also, it makes designing a fast-charging system easier, another reason for its popularity as a future anode material.

Before we go any further, you should know this about silicon: there are silicon and silicone. Of the two, the one that is often used for kitchen utensils is the synthetic polymer silicone that has a silicon-oxygen backbone. And what we will explore today is silicon.
Silicon is a metalloid with the atomic number 14 and symbol Si. It is the second most abundant element in the Earth’s crust by weight with 27.6% after oxygen. It is found as silica or silicate in nature and is one of the major components of sand and earth.

Earth and sand containing silicon have been used in architecture since the ancient times. Because silicon was so strongly linked with oxygen on Earth, it had not been recognized as an element until the 1800s. In 1787, French chemist Antoine Lavoisier first identified silicon but had no means to isolate the element at that time and described silica, an oxide of silicon, as an element. Later in 1824, Swedish chemist Jöns Jakob Berzelius succeed in isolating it.

The name silicon was given by Scottish chemist Thomas Thomson. He combined silex, the Latin word for flint, and “-on” because it is closely related to boron and carbon.
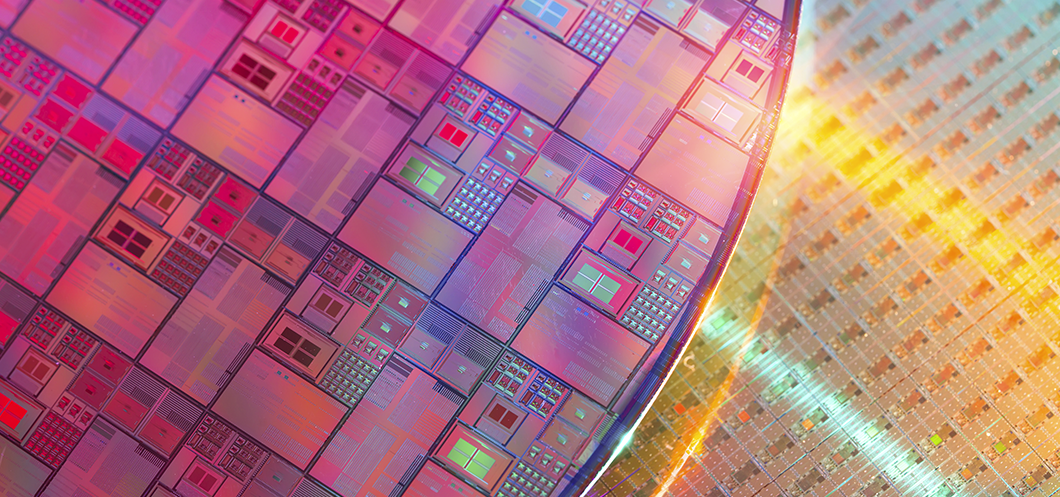
Silicon has been an important material in the semiconductor industry since the late 20th century for it has metalloid properties, is easy to supply, and is environmentally friendly. It is a common ingredient in the disc-like wafer, a thin slice of semiconductor.

Now, the element is drawing attention also in the battery industry as EVs have become popular. The EV market needs to improve the battery’s charging speed and driving range to grow further, and silicon is the right solution. Its demand in the anode material market is forecast to surpass that of natural graphite in 2027.
However, silicon is not without disadvantages as an anode material. It has issues like the volume expansion that happens during lithiation and can cause the silicon anode to break. Accordingly, research is going on to solve the problem, using it along with graphite and graphene. It will be interesting to see if silicon will be all the rage also in the battery industry after its success in the semiconductor industry.





