We see aluminum in our daily lives. Both the aluminum foil we often refer to as “tin foil” and use in the kitchen as well as beverage cans we buy at stores are made of aluminum. The metal is also used for lithium-ion batteries. Today, we will examine aluminum’s properties and uses.

Properties
Aluminum is a soft silvery white metal with the symbol Al and atomic number 13. The name originates from the Latin word “alumen,” meaning alum. Light, highly ductile, and malleable, aluminum can be easily extruded to take various forms. It also has high thermal and electrical conductivity. Pure aluminum forms a thin oxide layer in contact with the air, a process named passivation. The layer protects aluminum from an internal corrosion, although that may take the luster off the metal.

Refinement
Aluminum is the most abundant metallic element and the third most abundant element in Earth’s crust after oxygen and silicon. It once existed only in the form of oxides because of its high affinity to oxygen. The metal can be obtained from bauxite that is composed primarily of aluminum hydroxide. However, in the past, aluminum purification cost so much that the metal was called “the silver from clay.”

The price dropped after the Hall–Héroult process, the electrolytic smelting method was developed in 1886. American chemist Charles Martin Hall and French chemist Paul Louis Toussaint Héroult conducted research separately but discovered the electrochemical smelting process almost at the same time. It is interesting that the two entirely unrelated people born in the same year discovered the aluminum smelting method in the same year, isn’t it?
Various Uses of Aluminum
Aluminum is a useful raw material in many industries for its properties. It can be easily molded into various shapes as needed like foils and wires. The metal’s thermally and electrically conductive property makes it a good material for high-tension cables or kitchen utensils. It is also used for beverage cans, airplanes, ships, and vehicles due to its lightweight, durability, and corrosion resistance. In addition, aluminum is highly recyclable. Since recycling aluminum demands less energy and is more efficient than producing it, many countries encourage recycling the metal.

Aluminum makes our life more convenient with its versatility. Now it plays a crucial role in lithium-ion battery manufacturing. It boosts output of batteries as a cathode material in ternary battery NCA and quaternary battery NCMA. Moreover, it is used as an important cathode current collector in the battery electrode manufacturing process. In the coating process where binders are evenly dispersed onto the electrode, the cathode slurry is thinly coated onto aluminum foil.
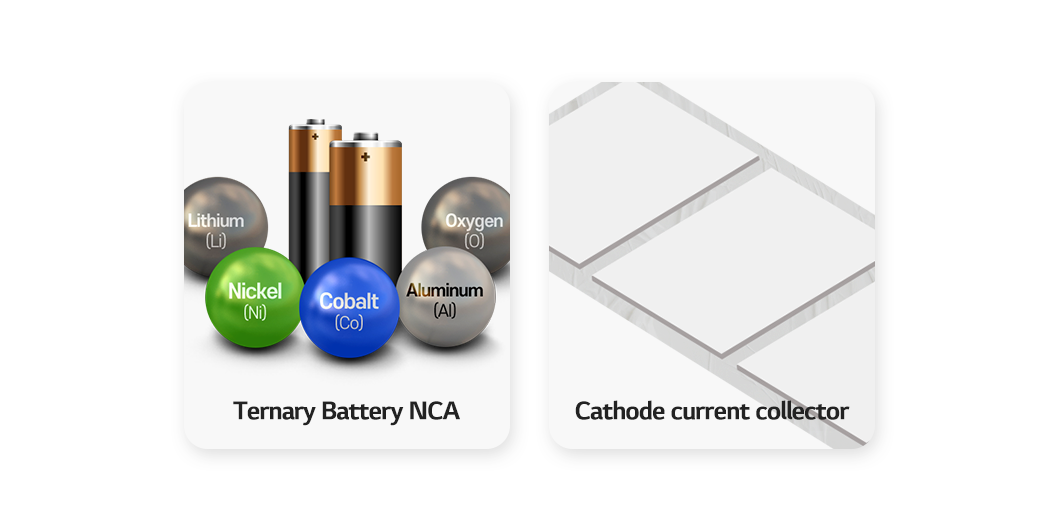
Since demand for lighter high-performing batteries is increasing with the popularity of EVs, use of aluminum is expected to only grow. The metal has made our lives more convenient in many areas. Now we can look forward to its role, especially in the battery industry.”





