A black, flaky graphite and a brilliant, hard diamond have entirely different looks, but they are both allotropes of carbon. Among the two, graphite is very common. Everyone knows it is contained in a pencil, but these days, it can be easily found in another item: the lithium-ion battery.
Then, how different is graphite from diamond and what characteristics make it a good ingredient of a battery?

Like diamond, graphite consists only of carbon and is soft with a hardness of 1.5 and a density of 2.23. The glossy black or iron-gray mineral is the perfect material for pencil as its name suggests; the word graphite comes from the Greek verb graphein, “to write.”

Although both graphite and diamond are composed of pure carbon, their different crystal structures yield different properties from each other. Diamond has a close-knit, dense structure in which each carbon atom joined to 4 other carbon atoms. The strong bond contributes to the high strength of diamond.

In graphite, each carbon atom is bonded with 3 other structures, forming a thin plane. The bond between the planes is easily breakable, but the layer structure makes graphite a good ingredient of the anode of a lithium-ion battery. The role of the anode is storing and releasing lithium ions arriving from the cathode (positive electrode) to generate power. The uniform layers offer a good condition for lithium ions to be stored between them.
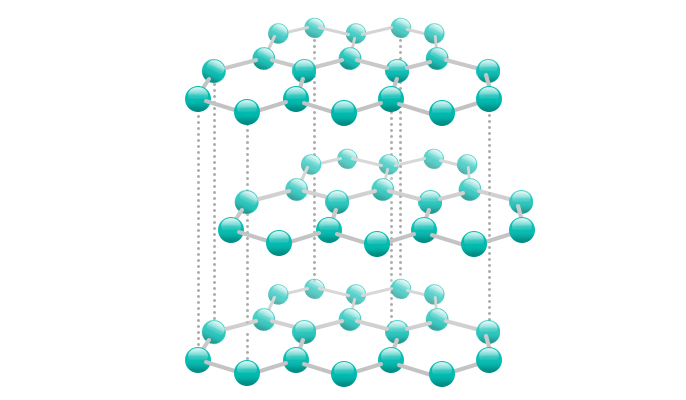
However, graphite expands and swells slightly when containing lithium ions. As the battery is repeatedly charged and discharged, the structure of graphite gradually changes, undermining the battery’s capability to store lithium ions and reducing battery life eventually. Graphite normally has a shorter life than the cathode, and the anode is one of the key factors that determines battery life.
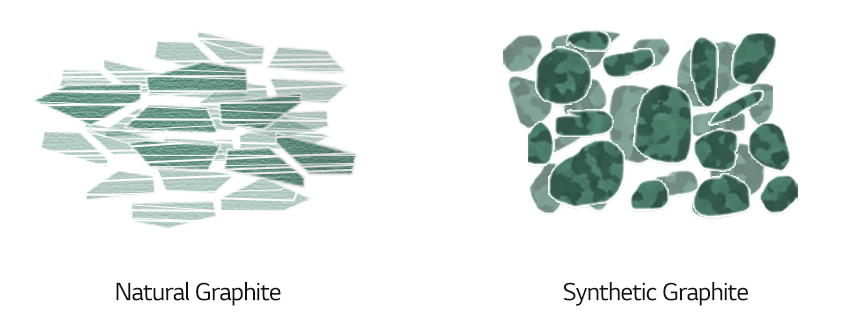
Replacing natural graphite with synthetic graphite for the anode can solve the problem. Synthetic graphite is manufactured by applying heat of above 2,500°C, a treatment for producing a stable and uniform internal structure of graphite. It can extend battery life since it is less prone to structural change after charging and discharging. Also, it can shorten charging time as it has more paths for transportation of lithium ions.
We have investigated about graphite, one of the major anode materials, in this session. As demand for a high-capacity battery grows, the industry is looking for next-generation anode materials that help fast charging. We will examine one of promising candidates, silicon, next time.





