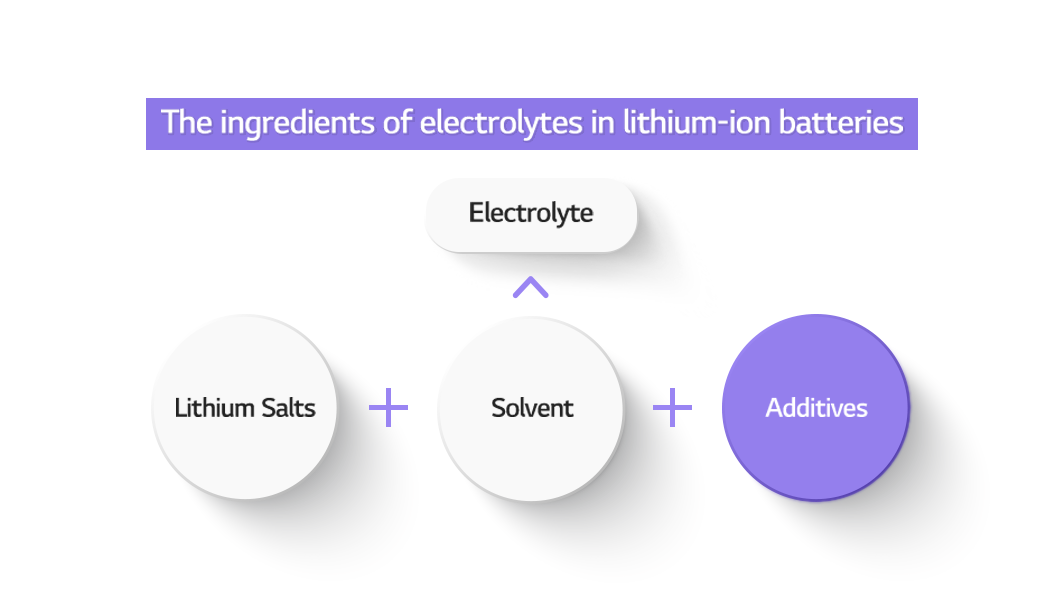
The electrolyte in lithium-ion batteries helps lithium ions shuttle between the cathode and anode. Among electrolyte components, electrolyte additives can make a significant difference with only a small amount. Today, we will talk about electrolyte additives for EV batteries.

Key functions
The main role of the electrolyte in lithium-ion batteries is to help the lithium ions shuttle between the cathode and anode. Electrolyte additives are a substance added to electrolyte in small quantities, but they protect the surface of the electrode for safer charging and discharging, or the movement of lithium ions. Therefore, they can contribute to better performance and safety of batteries.
Types of Electrolyte Additives
The most popularly used electrolyte additive for lithium-ion batteries is LiPF6. For EV batteries, about 5% of LiFSI (F electrolyte), LiPO2F2(P electrolyte), LiDFOP (D electrolyte), and LiBOB (B electrolyte) are added to the LiPF6 for higher performance and longer life. Since each of them has different properties, the type and arrangement of the additives in use have different effects on battery performance.
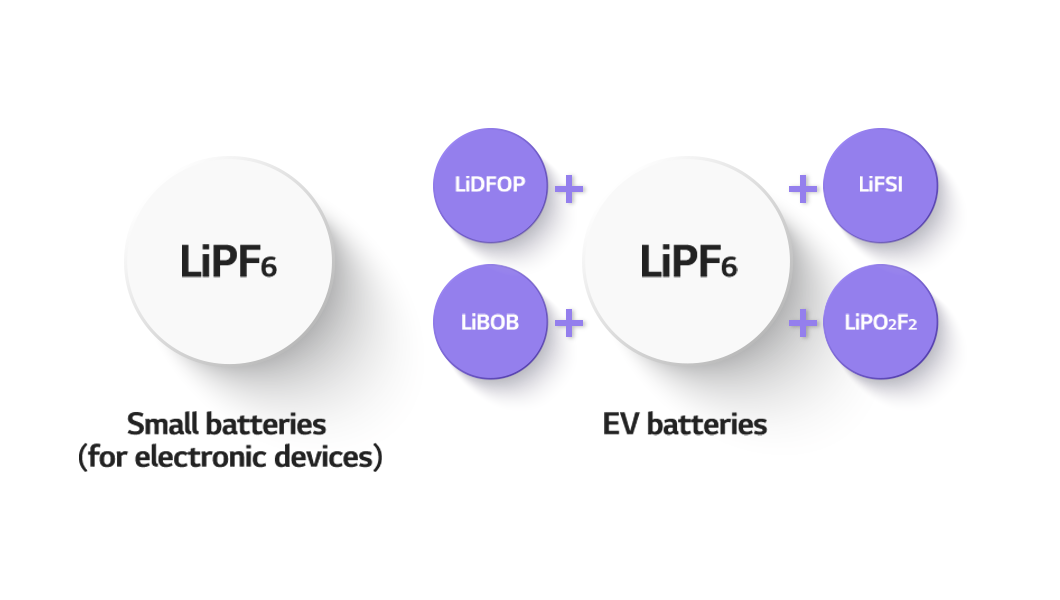
Electrolyte Additives for EV Batteries
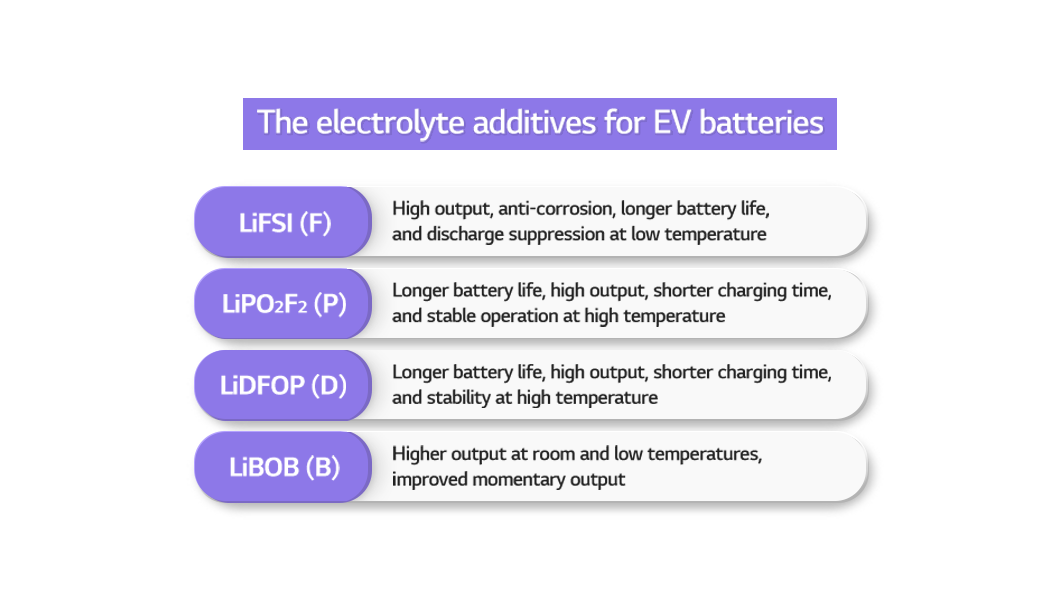
As for each of their notable features, the F electrolyte improves output capabilities and extends battery life by inhibiting corrosion. Also, it helps prevent discharge at low temperature. The P and D electrolytes extend battery life, shorten charging time, and help stable operation of batteries at high temperature. The B electrolyte improves output at room and low temperatures and increases the momentary output.
Performance and stability are highly important in a battery. Accordingly, researchers continue to look for better electrolyte additives since they can expect a strong effect even with a small quantity of them. Clearly, electrolyte additives have a superior capacity.





