Yang-Kook Sun
Professor, Department of Energy Engineering at Hanyang University
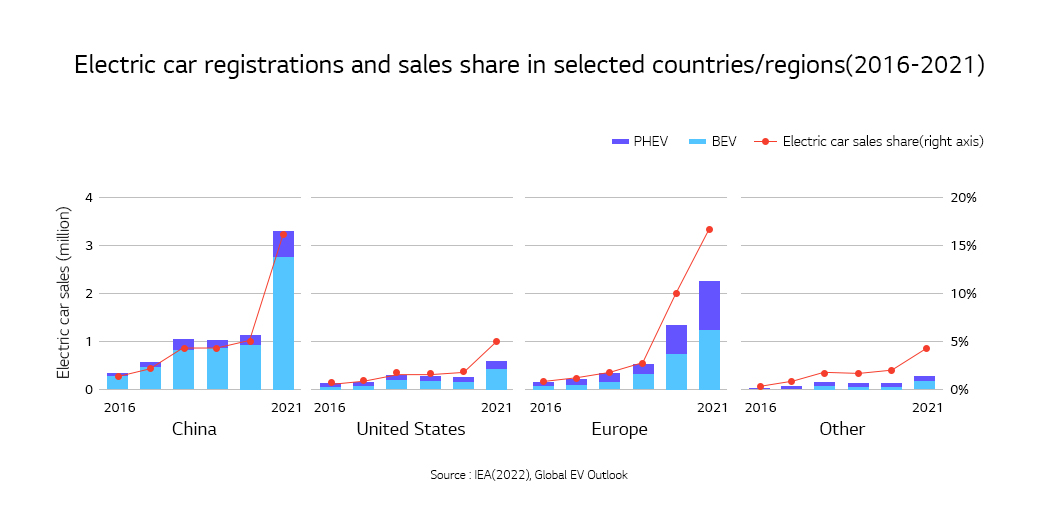
Sales of electric vehicles in 2021 doubled from a year earlier, accounting for almost 10% of the global car sales. The rise continued in 2022, recording 2 million global sales in the first quarter, up 75% compared to the same period of 2021. Also, a variety of EVs are seen more often on the road, indicating a rapid growth of the market.
The expansion of the EV market prompted the industry, academia, and research institutes to develop new materials for producing lithium-ion batteries that meet requirements like larger capacity, longer life, and low price. Among many components of lithium-ion batteries, the cathode takes up 40% of the battery price and is a key that determines battery capacity, life, and stability. Currently, the focus of development is on the layer-structured Li[NixCoyMn1-x-y]O2 (NCM) and Li[NixCoyAl1-x-y]O2 (NCA) cathode materials since they have high theoretical capacity (~ 280 mAh g-1) and high operating voltage (3.6 V vs. Li/Li+).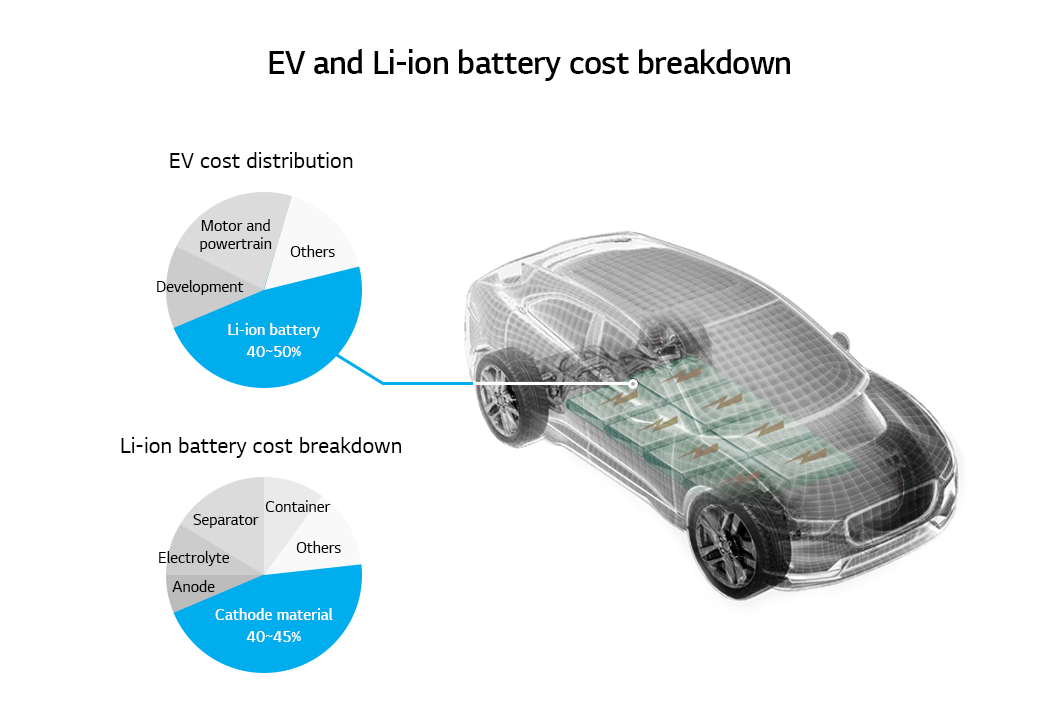
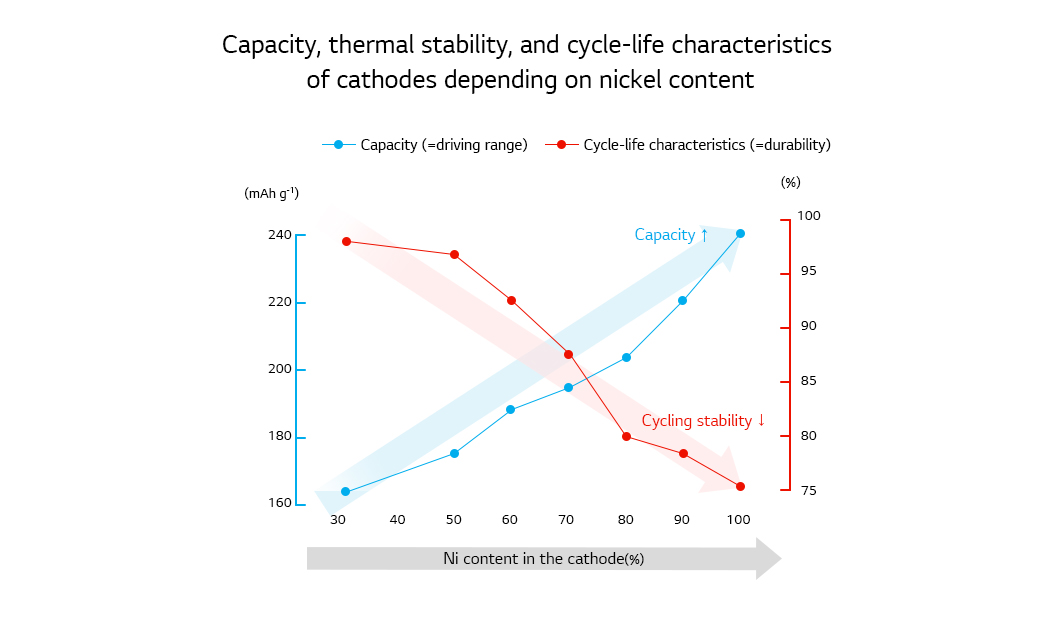
Driving range, one of the most important factors in EV performance, is determined by the energy density of the batteries installed in the vehicle. And the energy density is impacted by the usable capacity of the cathode. Higher nickel content in NCM and NCA cathodes raises usable capacity, which contributes to improving energy density, therefore research on increasing nickel content is going on. However, higher nickel content also has disadvantages: Cycling stability and thermal stability fall drastically. Indeed, this is an issue that needs to be resolved first in developing high-nickel NCM and NCA cathodes.
The main reason for the rapid performance degradation is that the unstable Ni4+ ions can induce side reactions when they meet electrolyte. High-nickel cathodes are vulnerable to the stress created inside the cathode particles during charging. The stress causes microcracks that serves as a path for the electrolyte to infiltrate into the cathode. Inside the cathode, the electrolyte cause side reactions across the board as well as the outside of the particle. Indeed, microcracks are the main culprit that contributes to the capacity fading of high-nickel cathodes upon repeated charging and discharging. Furthermore, the higher the content of nickel in the cathode, the more likely it produces serious microcracks inside the cathode particles.
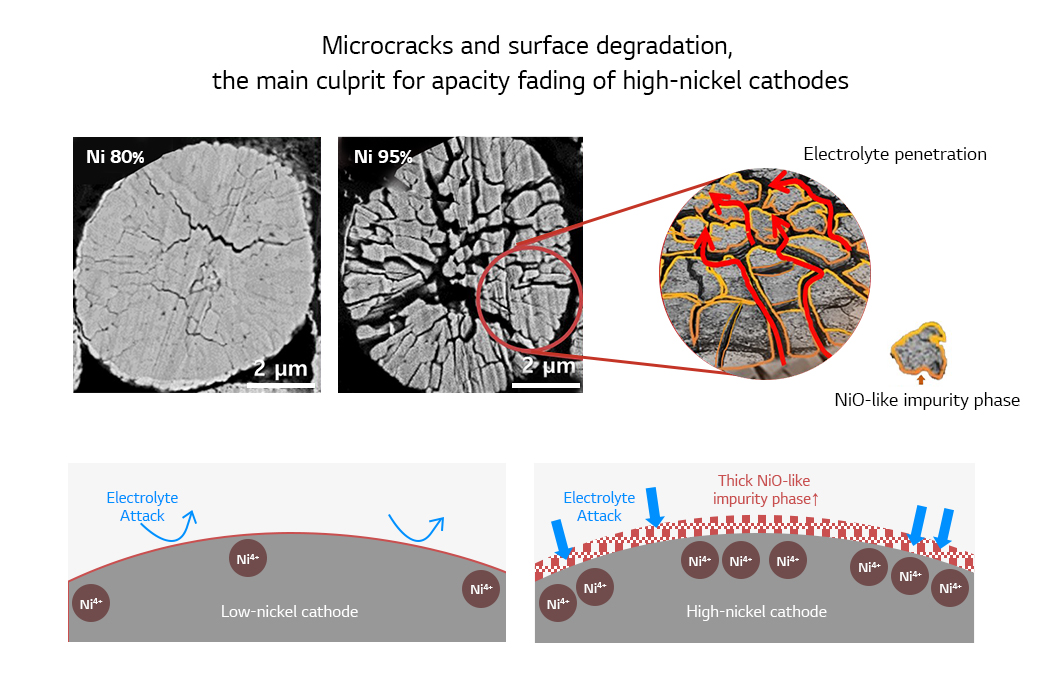
Accordingly, the focus in developing a high-nickel cathode that has high capacity and stability as well as good cycle-life characteristics needs to be on restricting the creation of microcracks and reducing surface reactivity. A remarkable result is the concentration gradient cathode in which the nickel content is high while manganese content is low at the center and vice versa at the edge of the particle. This cathode offers both high capacity and stability as its high-nickel center responsible for high capacity is wrapped with the stable low-nickel shell part. The low nickel content on the surface helps reduce reactivity to electrolyte and the unique microstructure helps disperse the stress created in the particle effectively, restricting the creation of cracks. The excellence of the concentration gradient cathode is recognized to be used for cathodes of EV batteries.
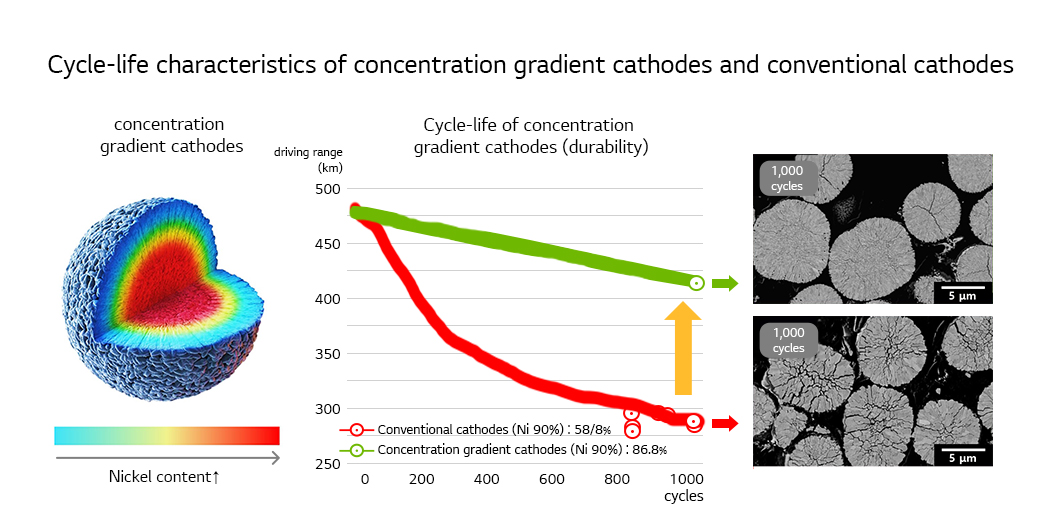
Besides increasing nickel content in NCM and NCA cathodes, going cobalt-free is gaining momentum.
Cobalt is one of the important materials for producing cathodes that take up the largest share of the cost of EV battery and its price is skyrocketing with the soaring demand for batteries. About 60% of the world’s cobalt reserves are in the Democratic Republic of Congo and most of them mined and primary processed in the country are exported to China that accounts for 50% of the global cobalt market. This suggests a possibility of supply instability for the rechargeable battery industry. IW-Consult, a German economic institute, warned that we would need a battery capacity of 1,300 GWh to produce 36 million new electric vehicles by 2030 but the known cobalt reserves would be enough to meet the predictable demands only for 11 years. Amid the accelerating transition from internal combustion engines to electric vehicles, the battery industry is cutting the use of cobalt for raw material security and price affordability.
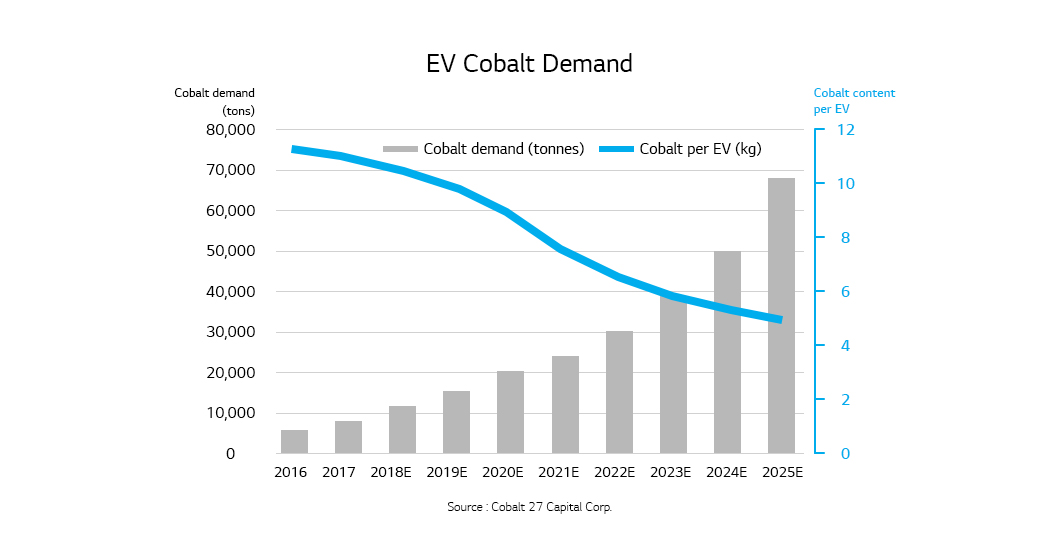
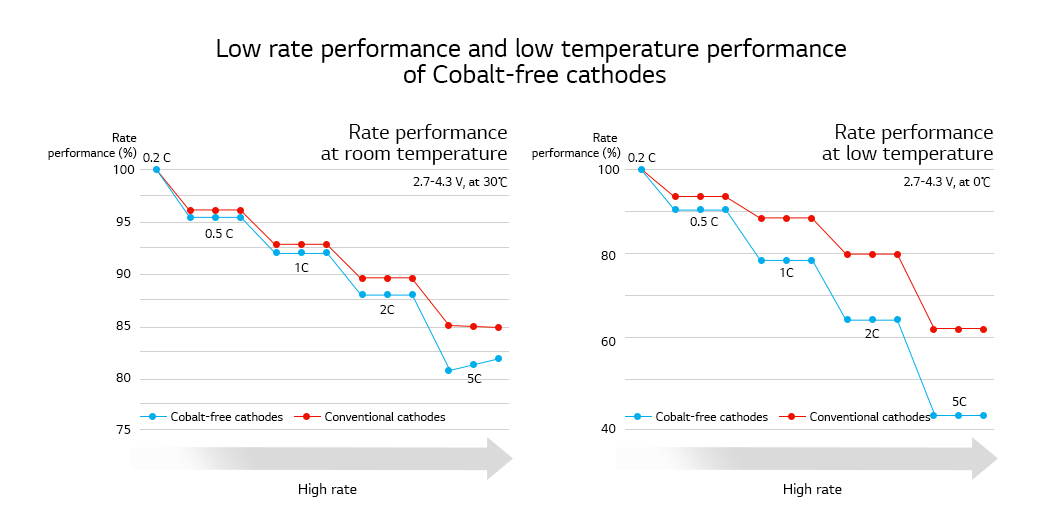
Going cobalt-free is essential for stability of lithium-ion battery prices and resource. However, it can bring about lower energy density, poor low-temperature performance, and poor rate performance since cobalt keeps the cathode structure stable and improves the rate performance in the cathodes of lithium-ion batteries. Therefore, the goal is to develop a cobalt-free cathode that works as good as NCM or NCA cathodes without sacrificing the advantages of using cobalt. To achieve the goal, a comprehensive understanding of cathode’s basic characteristics should come first and then strategies to overcome the disadvantages of going cobalt-free should be established based on the understanding.
*Rate performance: A capacity performance impacted by charging and discharging speed (charging and discharging current). A cathode with high rate performance shows high capacity even in rapid charging and discharging speed.
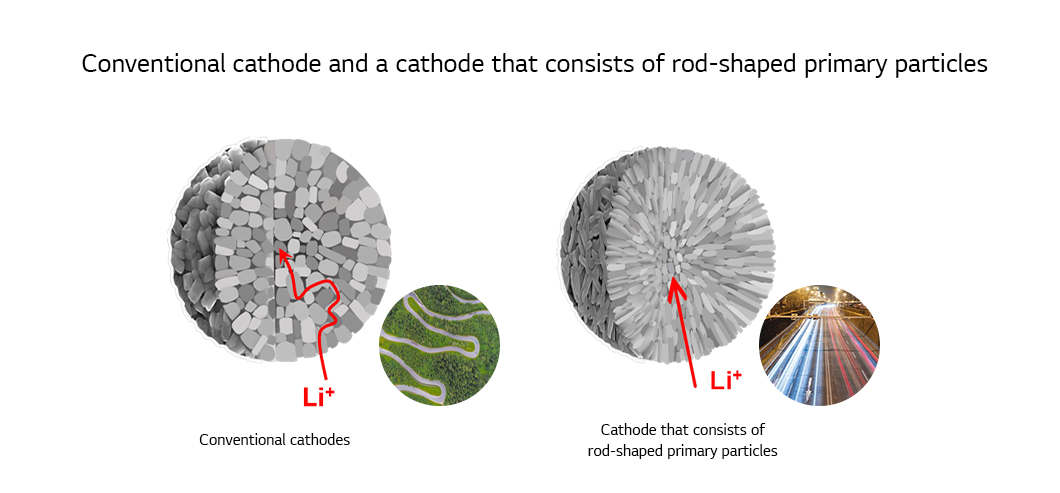
Various doping and coating methods used for NCM and NCA cathodes can be employed to make cobalt-free cathodes commercially viable. Since normal methods does not fit well in addressing the fundamental issues, development of a new technique is underway to allow lithium ions move smoothly by fixing the microstructure inside the cathode particle and improve the low rate performance of cobalt-free cathodes. A cathode with a microstructure of a radial arrangement of rod-shaped primary particles can form paths for easy travel of lithium ions, a feature that enhances rate performance. The structure also improves cycle-life characteristics by effectively dispersing internal stress and restricting creation of microcracks. As such, disadvantages of going cobalt free can be overcome by adopting various strategies to develop cathodes that have price stability and provide resource stability.
The goals of developing lithium-ion battery materials include not only achieving better performance but also meeting commercial demands and considering environmental circumstances. The battery industry has a potential for further growth. But it is especially sensitive to the changes in the market environment of electric vehicle industry, a downstream industry, and policies of each country as well as is witnessing rising and diversifying demand. Accordingly, the industry needs to meet requirements like being high energy, stable, high performing, and eco-friendly. Since these characteristics are mostly determined by the cathode, a key component of lithium-ion batteries, going high nickel and cobalt free will be even more important.
※ This column was written based on the writer’s views and does not necessarily reflect the opinion or strategy of LG Energy Solution.





