We are truly in the era of “cordless electronics.” Smartphones, tablets, and laptops have become essential parts of our lives. We see people listening to music via wireless earbuds on the street. Most households have a cordless vacuum, and the market is showcasing a variety of cordless electronics ranging from fans, hair dryers, and blenders to portable televisions. What led to the lifestyle change? It is mainly attributable to the development of lithium-ion batteries.
Lithium, the key material of the batteries.

In 1817, Swedish chemist Johan August Arfwedson discovered an element that fizzes and explodes in contact with water as he was analyzing the mineral petalite. It contained about 4% alkali metals that were previously unknown. The new element was named from the Greek word “lithos” meaning stone, because it was found in a mineral as opposed to other common alkali elements (such as sodium and potassium) which had been discovered in plants. At that time, purifying lithium was not possible because of the lack of electrolysis machine. But in 1818, British chemist Sir Humphry Davy first successfully isolated lithium.

Lithium’s atomic number is 3 and the symbol is Li. An alkali metal that belongs to the Group 1 of the periodic table, its atomic mass is 6.941, melting point 180.54℃, boiling point 1,347℃, and density 0.534. The silvery white metal is the lightest metal with the lowest density of all solid elements, meaning it is soft enough to be cut with a knife. As lithium reacts easily with oxygen or water like other alkali metals do, it is found only in compounds in nature. In addition, since lithium reacts violently with water like when Arfwedson first found it, it is normally stored in oil such as paraffin wax.

Although lithium is being used for many purposes, its role is especially pronounced in the battery industry. One of the most important materials of lithium-ion battery, currently the most common rechargeable battery, lithium is used for the cathode among the 4 major elements of lithium-ion batteries (3 other elements are anode, electrolyte, and separator). Lithium-ion batteries have the advantage of extra high energy density as well as fast charging and discharging. Also, they are lightweight and offer large capacity compared to nickel-cadmium or nickel-metal-hydride batteries. As a result, the lithium-ion battery has become the most popular battery.
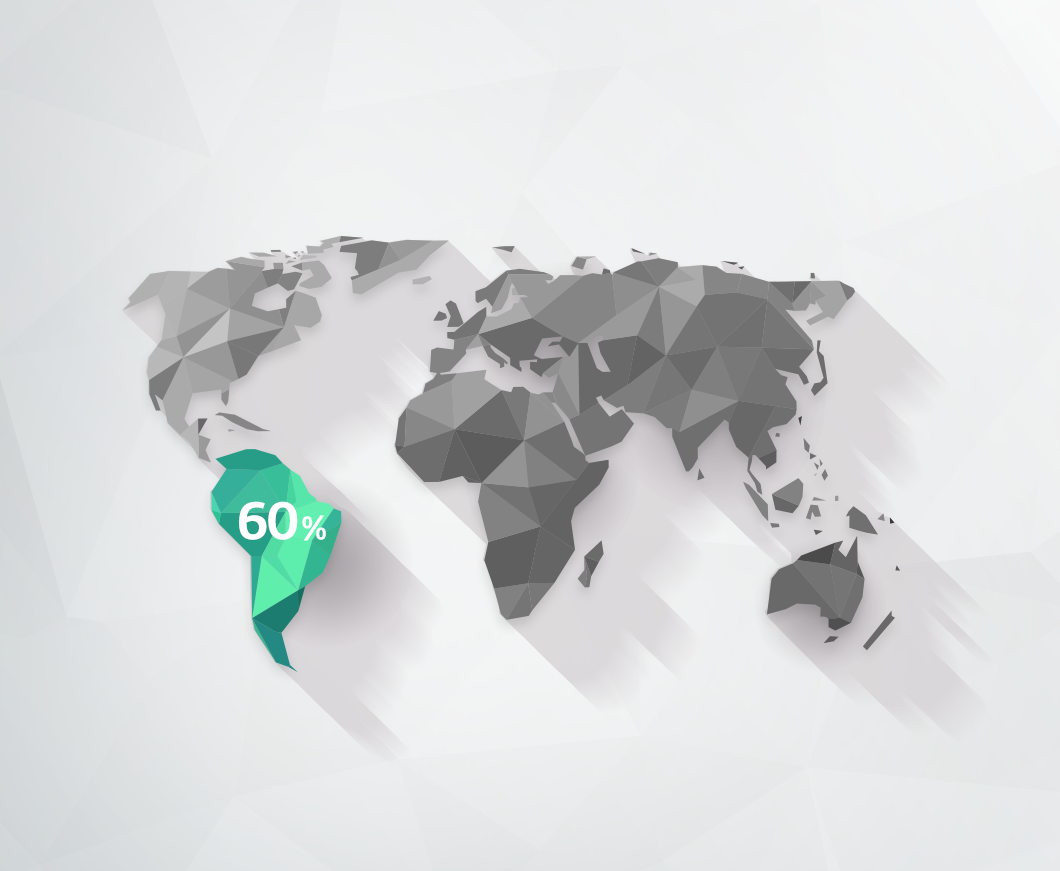
Lithium’s value has become even greater with the wider use of electric vehicles. The soaring lithium prices pushed countries to look for lithium further, eventually revealing more reserves every year. The largest lithium producers include Australia, China, the U.S., Chile, Brazil, Argentina, and Bolivia. Currently, Latin American countries are reported to hold 60% of the world’s lithium reserves.
As the battery industry is expected to be the next leader following the semiconductor industry, lithium’s value is rising so high that it is called “white gold.” We look forward to seeing how lithium will change the battery industry.





