One of the four components of batteries, the cathode determines the capacity and voltage of a battery. And to produce a cathode, a precursor is necessary. With the importance of the precursor growing, we will explore what it is and why it is gaining popularity.
What is a Battery Precursor?
The precursor, in producing material A through a chemical process, is a material at immediately before the final step of becoming material A. A battery precursor is a material at the final step before becoming a cathode, or an ingredient from which a cathode is formed.
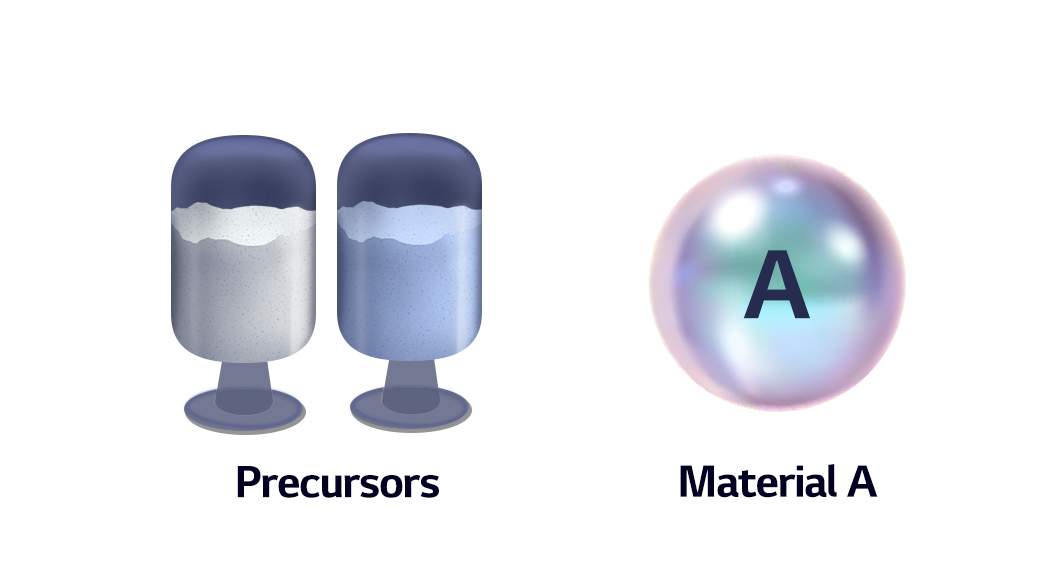
The performance and purpose of a battery are determined by which active materials are used for its cathode. Various combinations of cathodes can be made by adding metals in addition to lithium oxide, a basic ingredient. Commonly used metals include nickel, cobalt, and manganese. A chemical compound consisting of these materials is a precursor, which turns into a cathode with an addition of lithium.
How is a Precursor Produced?
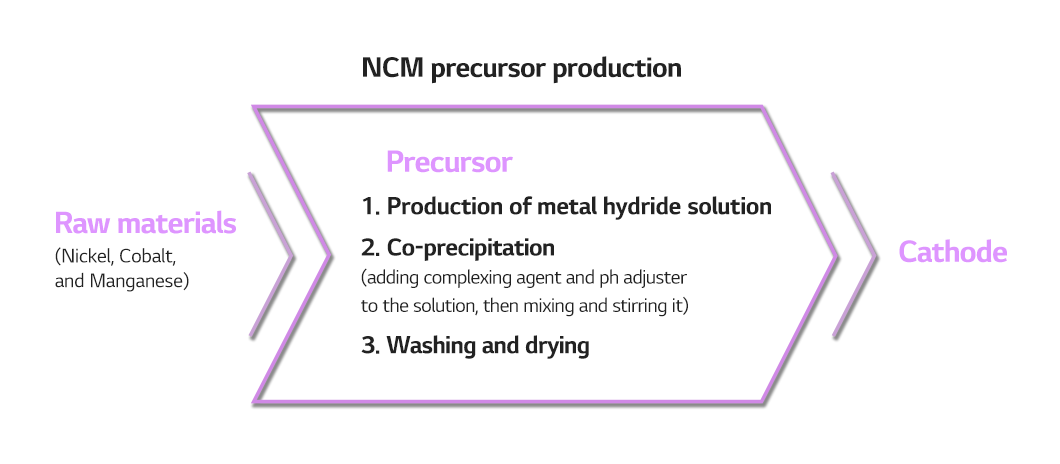
The most used method for producing precursor is co-precipitation, a process in which a substance carries down other substances along with it. Like the name suggests, it is the simultaneous precipitation of different ions in aqueous or nonaqueous solutions.
To take the example of an NCM precursor, the first step to make it is to dissolve nickel, cobalt, and manganese to make a metal solution. When complexing agent and pH adjuster are mixed into the solution and stirred, reaction and flocculation take place, inducing precipitation. The last is to wash and dry the precipitated substances.
A cathode can be made in different ways using different precursors. The three methods for producing NCA cathode materials in the figure below can help you understand better. NCA cathode materials consist of nickel, cobalt, aluminum and lithium oxide. But each process and form of precursors differ slightly.
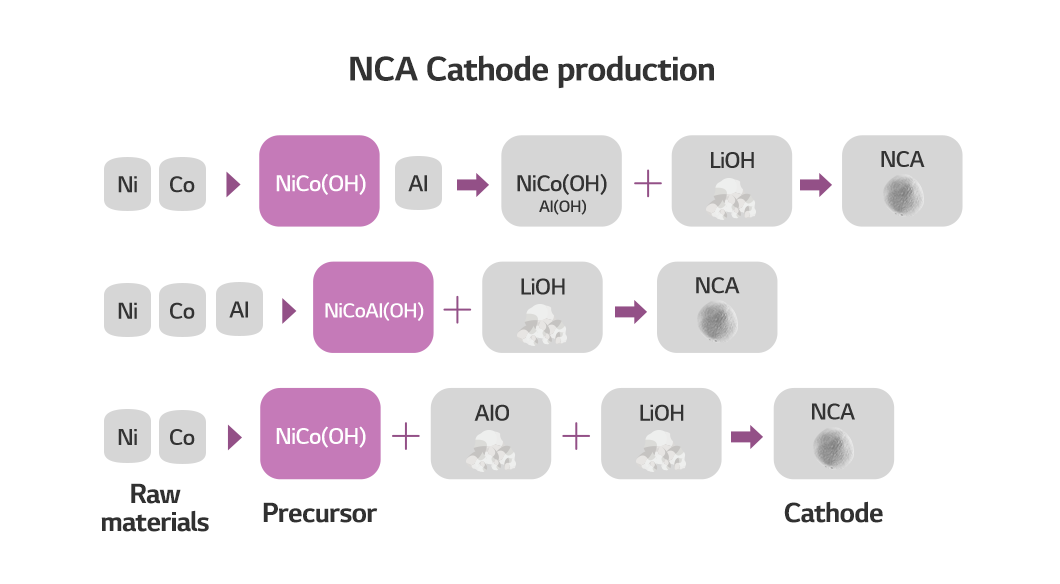
While nickel, cobalt and aluminum are all mixed in the second, the first and third precursors are made with nickel and cobalt. Also, even though they share the same two metals as ingredients, aluminum is added and coated in the first, unlike in the third. As such, the production method differs according to the precursor.
Classification of Precursors, Secondary Particles and Primary Particles
Precursors can be classified into secondary and primary particles depending on the size. Secondary particles are the larger ones with the size of 10-20 μA and primary particles are those with below 5 μA. Rising demand for high-capacity, high-output batteries is leading the growth of primary particle production. Precursors with smaller particles can store more energy and prompt faster electrochemical reactions since they have larger contact surface areas. Normally, they are used together, with smaller primary particles filling up the spaces created between the larger and cheaper secondary particles, to improve density and stability.
Why Localization of Precursors is Necessary
Precursors are important in battery manufacturing, taking up 70 % of the cathode material costs. As the EV market continues to expand, Korean battery makers seek to develop their own technology of producing precursors in order to reduce dependence on imports and stabilize supplies. They are currently concentrating on boosting energy density and stability to prevent an explosion. Since Korean companies have begun research to develop their own technology in earnest, we might soon see “K-precursors” becoming globally popular.





