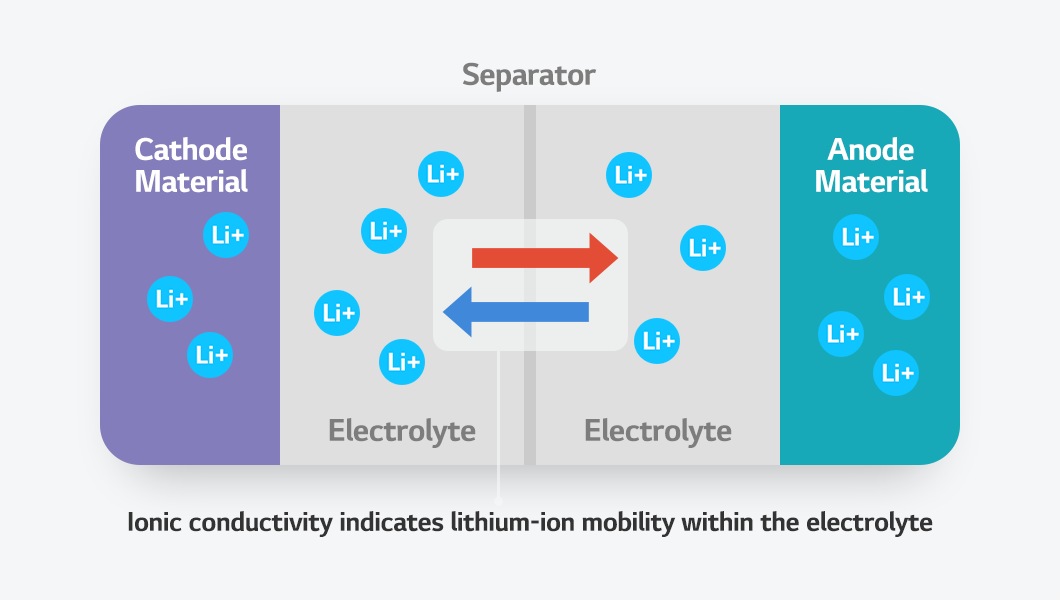
In a battery, lithium ions travel between the cathode and anode during charging and discharging. During charging, lithium ions move from the cathode through the electrolyte to the anode, where they are stored. During discharging, they move in the opposite direction, returning to the cathode from the anode and generating an electric current. The indicator that reflects how smoothly lithium ions move is ionic conductivity. In this Battery Glossary, we will explore what ionic conductivity is!
*View Lithium-Ion Battery’s Structure and How It Works

What Is Ionic Conductivity?
Ionic conductivity is an indicator of the ease of lithium-ion movement within a material to generate electricity. In batteries, it refers to the degree of lithium-ion transport through the electrolyte, which is essential for basic operation. Ionic conductivity varies depending on factors such as the ion concentration, the amount of electric charge, and ion mobility in the electrolyte.
Importance of Ionic Conductivity in Batteries
Ionic conductivity plays a crucial role in evaluating battery performance and efficiency. The electrolyte acts as the pathway through which lithium ions move between the cathode and anode. The higher the ionic conductivity, the lower the electrolyte’s internal resistance, enabling high-output, high-speed charging and discharging. Conversely, when ionic conductivity is low, ion movement slows, reducing charging and discharging speed and causing a drop in voltage. This eventually leads to lower battery output and a shorter lifespan.
In addition, ionic conductivity is significantly affected by temperature. When the temperature rises, ions move more actively, increasing ionic conductivity. When the temperature drops, ionic conductivity decreases. As a result, battery performance declines in cold environments, whereas charging and discharging operate more smoothly in warmer conditions.

So, what are the conditions for electrolytes with high ionic conductivity?
An ideal electrolyte allows ions to pass smoothly while blocking electrons. It is also essential that it has high ionic conductivity and a negligible level of electronic conductivity at the same time. The liquid electrolytes currently in use support fast charging and discharging, as well as high current flow, because ions can move freely within them. Meanwhile, solid electrolytes used in solid-state batteries—considered next-generation technology—have more limited ion-transport pathways compared to liquid electrolytes. This is because solids have higher density than liquids, and ions must move through a crystal lattice, which restricts their movement. Accordingly, achieving ionic conductivity in solid electrolytes that is comparable to that of liquid electrolytes at room temperature is a major challenge in developing next-generation batteries.
*What is electrolyte? View Electrolytes for Lithium-Ion Transport
Electrochemical Impedance Spectroscopy (EIS) for Measuring Ionic Conductivity
EIS is a common method for measuring the ionic conductivity in battery electrolytes. It works by applying a small alternating-current (AC) signal to the electrolyte and analyzing the resulting electrical response to calculate electrolyte resistance. In particular, using an electrode that allows ions to pass while blocking electrons makes it possible to determine ionic mobility in the electrolyte based on the measured resistance.
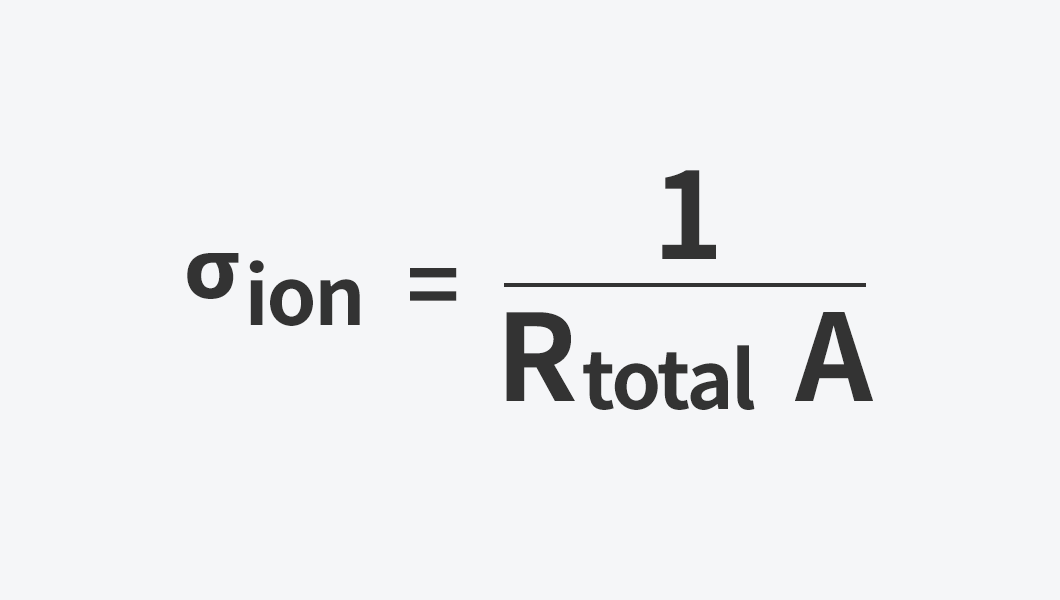
Using the obtained resistance (R), sample thickness (l), and electrode area (A), ionic conductivity can be calculated with the following equation.
This method is widely used in battery research because it enables separate identification of the electrolyte’s internal resistance and the electrode–electrolyte interfacial resistance.
As such, ionic conductivity is an essential concept to understand for improving battery performance, and various research efforts are underway to enhance it in next-generation batteries. We will continue to explore basic battery concepts in an easy and engaging way in our next session.