If the cathode material is where lithium ions in a lithium-ion battery are released, the anode material is the space that stores the lithium ions from the cathode. So, the anode material performs certain functions within the battery and is made up of various substances. Let’s take a closer look at what the anode material does within the battery and what materials are used for it.
Anode material affecting battery charging speed and lifespan
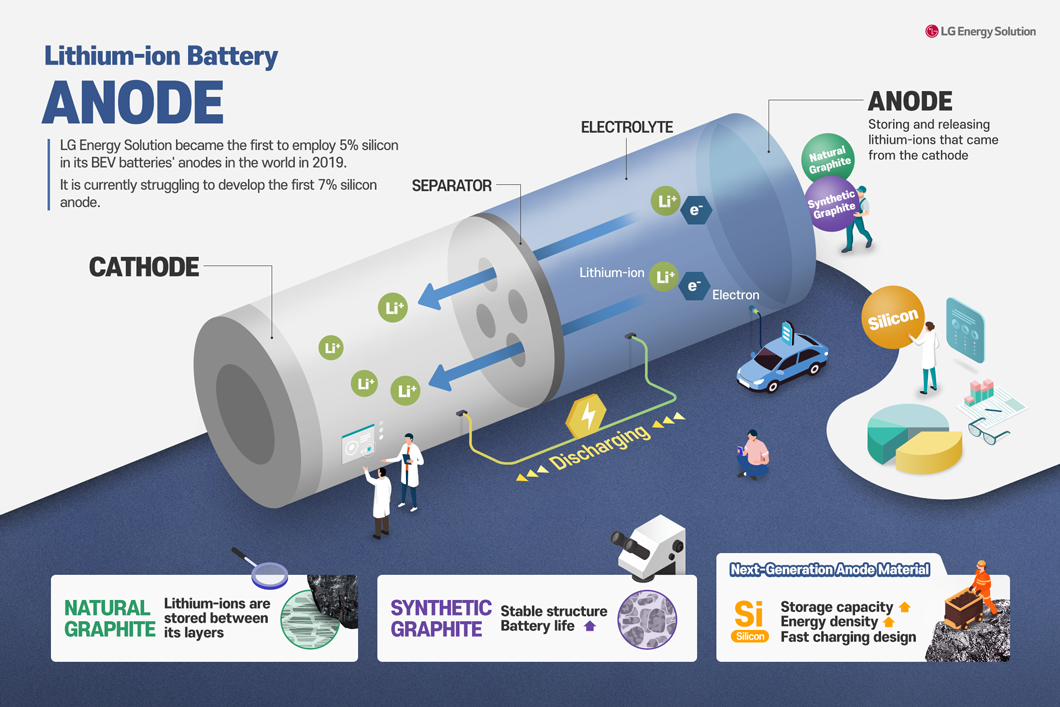
The anode enables the flow of current by storing and releasing lithium ions. The lithium ions in the anode material move through the electrolyte to the cathode material. The separated lithium ions and electrons travel along the conductor connecting the cathode and anode. Through these movements, electrical energy is generated.
However, the anode material is a factor that affects the battery’s charging speed and lifespan in this process. The greater the number of lithium ions that the anode material can store, the longer the battery lifespan, and the better the anode material can accept lithium ions, the shorter the charging time can be. Therefore, even if a significant amount of energy is generated in the cathode material, if the anode material cannot properly store lithium ions, the battery energy cannot be efficiently utilized. As a result, the significance of anode material technology development is continuously increasing.
Graphite, the Representative of Anode Material
“Graphite” is the most commonly used material in anode materials up to now. Graphite, consisting purely of carbon, forms thin layers with a repeating hexagonal shape where one carbon atom bonds with three other carbon atoms, creating a layered structure. Due to graphite’s regular structure, lithium ions are stably stored in between, making it suitable as an anode material.
Graphite is divided into natural graphite and synthetic graphite. When natural graphite is used as an anode material, it offers advantages such as high lithium-ion storage capacity and low production cost. However, natural graphite tends to expand slightly in volume when lithium ions enter. As a result, when repeating charging and discharging the battery, the structure gradually changes, leading to a decrease in the lithium-ion storage capacity and a reduction in the battery lifespan.
Silicon, the Emerging Next-generation Anode Materials
With the increasing demand in the electric vehicle battery market, the demand for next-generation anode materials is also growing. The material receiving attention as the next-generation anode material is silicon.
If silicon is used as an anode material instead of graphite, the energy density per gram of the battery increases by more than four times, making the design for rapid battery charging easier. Furthermore, silicon is considered an environmentally friendly material, which is why it is garnering attention as the next-generation anode material.
LG Energy Solution is also putting significant efforts into the development of silicon anode materials. In 2019, they achieved a groundbreaking accomplishment by applying silicon anode material with a 5% content to BEV batteries for the first time globally. Additionally, we are continuously driving technological innovation by accelerating the development of technology for applying silicon anode materials with 7% content, another world-first achievement.
Up to this point, we’ve explored the role of the anode material, a crucial component in lithium-ion batteries, as well as the characteristics of two materials commonly used for anodes: graphite and silicon. In our next session, we will continue to provide insightful and easily understandable information about batteries through infographics!





