Coins have been in human history for a very long time.
They are mostly made of copper and in Korea, even the word dongjeon (meaning coin) refers to money made of copper. Although the use of coins is increasingly declining, copper is expected to remain an important element for humans as it is becoming a key ingredient for batteries.

Humans first used copper c. 8,000 B.C.E. It has been widely used for a long time since it is found in a directly usable metallic form in nature. The introduction of copper contributed to development of quenching and smelting techniques, the birth of bronze, a copper-tin alloy, and the establishment of the iron-smelting technique. As such, it has made a notable impact on human history.

Copper’s atomic number is 29 with the symbol Cu. It is the 25th most abundant element in Earth’s crust and one of the most recycled metals after steel and aluminum. In Roman times, copper supply came almost entirely from Cyprus. So, it was called cyprium, meaning “a metal of Cyprus” and later shortened to cuprum, hence the English copper.

Copper is soft, ductile, and malleable and therefore, can be stretched thin. A good conductor of electricity and heat, it gets hardened when alloyed with other metals. Such properties make copper a good ingredient for diverse products across the industry from items that require high thermal and electrical conductivity such as electric wires, kitchen utensils, electronic devices, EVs, and heat pipes to ornaments, ship propellers, wind instruments, door knobs, and valves. Furthermore, copper is biostatic, meaning it inhibits growth of an organism on it. Therefore, it is applied to pesticides and antimicrobials to kill harmful insects and inhibit the growth of fungus.

Another property of copper is that it oxidizes to form a green rust that is called verdigris (or patina) on the surface. Since the verdigris layer protects the underlying copper from further corrosion, it is often used for roofs of buildings. When the Statue of Liberty was unveiled in the U.S. in 1886, the landmark of New York City was not green. The current color is because copper, of which it is made, reacted with the air.
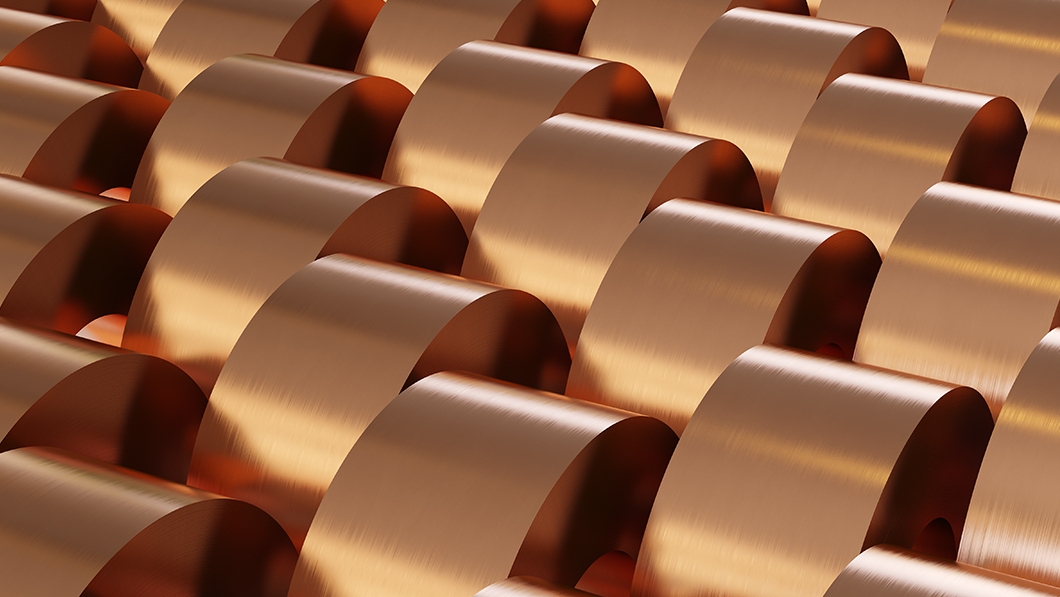
In the battery industry, copper is a popular anode ingredient. Elecfoil coats the anode of a battery and takes the role of a current collector that collects or supplies electrons in an electrochemical reaction. The current collector has to be as thin as possible for lightweight of the battery and regular for high capacity. Accordingly, a ductile, highly conductive copper was adopted to produce the anode current collector, the copper foil for batteries.
Copper has been a highly useful element to humans since ancient times. It is expected to continue to be so across the industry because the demand for EVs and electronic devices powered by batteries keeps rising.





