With the evolution of rechargeable lithium-ion battery technology, batteries have been equipped with higher energy density, which translates into a more extended range on a single charge for electric vehicles. However, since lithium-ion batteries generate electrical energy through the chemical reaction of lithium ions, no matter how high the energy density is, the battery range won’t extend significantly if the movement of lithium ions is not smooth. Today, let’s look into the electrolyte, a medium that facilitates the movement of lithium ions between the cathode and anode.

The 3 Major Components of Electrolytes
Lithium ions move between the cathode and anode through the electrolyte when a lithium-ion battery is charged or discharged. Think of it as the lithium ions commuting back and forth in a dedicated vehicle named electrolyte. The electrolytes in lithium-ion batteries are generally liquid with dissolved lithium salts. An electrolyte consists of salt, organic solvent, and additives.

① Lithium Salts
Lithium salts serve as pathways for lithium-ion movement. In other words, lithium ions dissolve in the salt, allowing them to travel freely. The most commonly used lithium salt in lithium-ion batteries is LiPF₆ (composed of lithium, phosphate, and fluorine), also known as a general-purpose electrolyte or general-purpose lithium salt. Thanks to its excellent ionic conductivity, solubility, and chemical stability, it is widely used in small IT devices such as smartphones. For EV batteries, LiPF₆ is often combined with additional lithium salts, including LiFSI (electrolyte F), LiPO₂F₂ (electrolyte P), LiDFOP (electrolyte D), and LiBOB (electrolyte B). These additives help extend battery lifespan, enhance charge-discharge efficiency, and mitigate battery discharge at low temperatures. Meanwhile, to improve low-temperature performance, a small amount of LiFSI lithium salt—known for its superior lithium-ion mobility in cold conditions—is sometimes added.
② Organic Solvent
The organic solvent helps dissolve lithium ions in salt. To perform this role, the solvent needs to have high permittivity to effectively separate ionic compounds and low viscosity for the smooth movement of lithium ions. However, a solvent with high permittivity tends to have high viscosity, and a solvent with low viscosity tends to have low permittivity. That is why chain carbonate with low permittivity and viscosity is mixed with the basic solvent, cyclic carbonate with high permittivity and viscosity, as a cosolvent to achieve balance and optimum ionic conductivity.
③ Additives
Additives are substances added in small amounts, typically less than 5%, to enhance battery lifespan, improve stability, and optimize performance. Since the electrolyte comes into contact with both the cathode and anode, additives can be introduced to affect both electrodes. Cathode additives primarily help mitigate heat generation, while anode additives form a protective layer that enhances durability and extends battery life. Although used in small quantities, these additives play a crucial role in electrolyte performance. Additives are used in appropriate amounts depending on the required performance of organic and inorganic compounds. In particular, inorganic additives mainly act on the cathode, helping to suppress gas formation inside the battery and reduce resistance.
Although added in small amounts, these additives play a crucial role in the electrolyte. These additives are used in appropriate amounts according to the performance requirements of organic and inorganic compounds. In particular, inorganic additives primarily act on the cathode, helping to suppress gas formation inside the battery and reduce resistance.
The electrolyte is made up of these three main components – lithium salts, organic solvents, and additives. These are injected into batteries at the final stage of the battery manufacturing process. Lithium ions are ready to travel when the electrolyte permeates the separator, the anode, and the cathode.
Conditions for Good Electrolytes

First, electrolytes must allow many ions to move in a short period of time. Ion conductivity, which is the efficiency of transporting lithium ions, needs to be fast to enable fast charging.
Second, electrolytes must be chemically stable. In other words, they should not cause unexpected reactions (side reactions) while the battery is operating. Recently, several additives are added to minimize harmful side reactions.
Third, electrolytes must be cold and heat-resistant. Since electrolytes are liquids, their performance may decrease when the temperature drops as molecular mobility slows down. So, the battery would work in any environment when its electrolyte has a low freezing point and high flash point.
Electrolytes vs. the Solid-state Battery Market
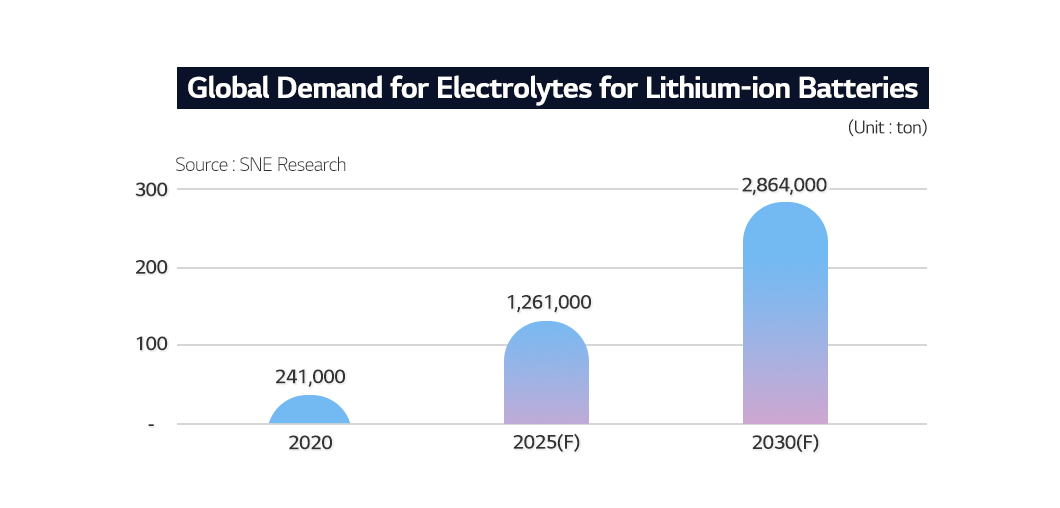
The growth of the rechargeable battery market is driving the demand for electrolytes. According to SNE Research, global electrolyte demand is expected to jump from 240,000 tons in 2020 to 2.86 million tons by 2030.
Recently, several companies have been researching solid-state battery technology using solid electrolytes. In theory, solid electrolytes can overcome the disadvantages of liquid electrolytes. As solid electrolytes are denser than liquid electrolytes, they would be more resistant to external shocks and more energy-efficient. A study points out that batteries with solid electrolytes can double the range of electric vehicles with batteries using liquid electrolytes of equal size. However, more research and development is required before commercialization.
How would solid-state battery technology change the electrolyte market? Experts say that solid-state batteries would be very expensive at the beginning of their commercialization. Manufacturers may not choose expensive solid-state batteries if liquid electrolyte technology could instead guarantee satisfying range and safety at a competitive price by then. Many eyes are on the development of electrolytes and the battery market, as batteries continue to evolve.





