
Demand for batteries is increasing across various sectors, including electric vehicles, energy storage systems (ESS), and electronic devices. Since each application requires different features, research is underway to develop new batteries using diverse materials. Amid this trend, one battery is drawing particular attention: the sodium-ion battery.
Here, we will examine the key features of sodium-ion batteries and LG Energy Solution’s current roadmap for their development.
What Is a Sodium-Ion Battery?
A sodium-ion battery is a type of battery in which sodium ions (Na⁺) move between the cathode and anode to store and release electricity. Its structure and operating principles are largely similar to those of lithium-ion batteries. But as the name suggests, the key difference lies in that sodium replaces lithium as the electrode material.
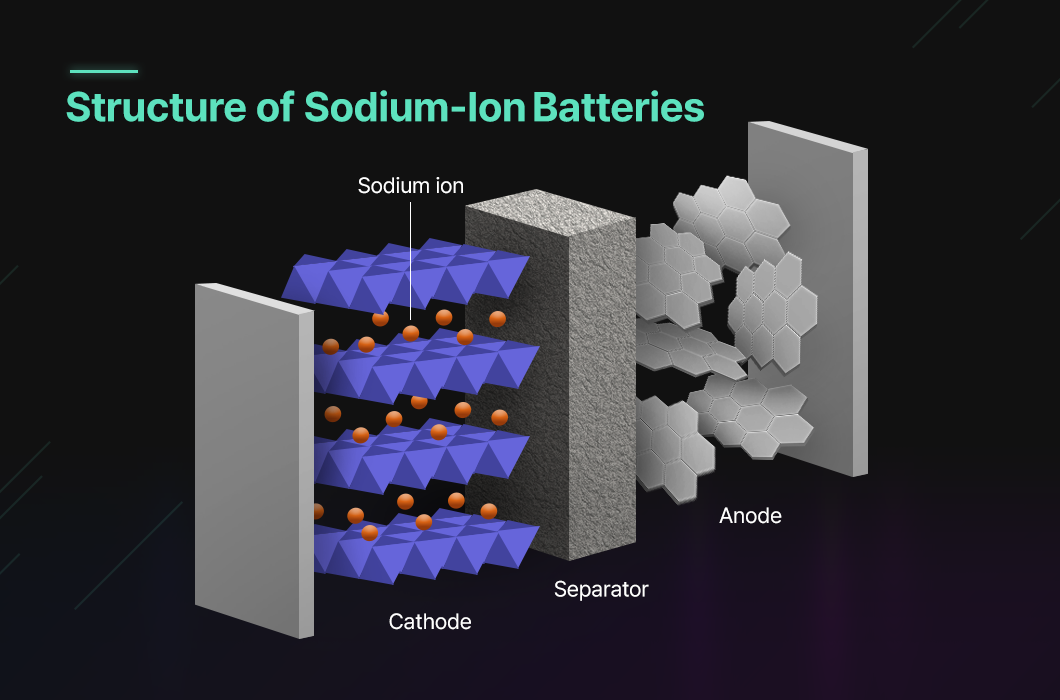
Looking at the structure of sodium-ion batteries, the cathode mainly consists of layered sodium transition metal oxides (NaTMO₂), polyanionic compounds, and Prussian blue derivatives. For the anode, carbon-based materials such as hard carbon and soft carbon are commonly applied. The electrolyte usually includes an organic liquid electrolyte containing dissolved sodium salts, which dissociate into sodium ions and anions in a state capable of electrochemical reaction. These ions shuttle between the electrodes, enabling current to flow.
Interest in sodium-ion batteries dates back a long time, with basic research beginning around the similar period as lithium-ion batteries. Sodium itself was identified in 1807, when British chemist Sir Humphry Davy first isolated the element through electrolysis. Later in the 1970s, researchers proposed inserting lithium ions into the layered structure of titanium disulfide (TiS₂) to induce electrochemical reactions, initiating efforts to apply this principle to batteries. In the 1980s, it was discovered that inserting sodium ions into the same TiS₂ structure also enabled highly efficient and reversible electrochemical reactions at room temperature. This finding demonstrated that sodium could likewise drive electrode reactions, marking the true beginning of sodium-ion battery research.
During the same decade, the electrochemical properties of lithium cobalt oxide (LiCoO₂) were first reported, prompting research on layered oxides (NaₓCoO₂) involving sodium. These studies confirmed the potential of sodium as a layered structure-based electrode material.
Strengths of Sodium-Ion Batteries: An Emerging Contender in Next-Generation Energy Storage
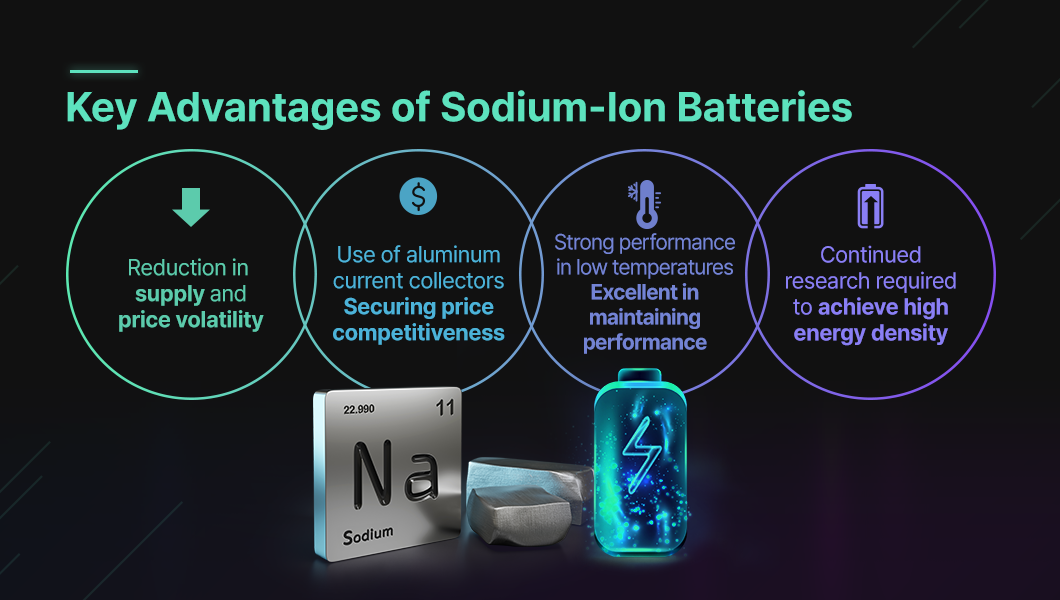
Sodium is the fifth most abundant element on Earth and is widely distributed across the world in the form of rock salt or seawater salts. Accordingly, its supply chain risk, manufacturing cost, and price volatility are all low.
Another property of sodium also enhances its price competitiveness. While lithium-ion batteries use copper as the anode current collector, sodium-ion batteries can use the more affordable aluminum, since sodium is not reactive with it.
In particular, sodium-ion batteries are known to maintain stable performance in low-temperature environments when certain electrode materials are applied. Research has shown that they retained about 90% of their initial room-temperature capacity even at –20 °C. By contrast, lithium-ion batteries under the same conditions dropped to around 60–70% of their capacity. As a result, the battery industry expects that applying sodium-ion batteries to electric vehicles could mitigate performance degradation in winter.
Lastly, sodium-ion batteries theoretically have the potential to achieve high energy density. Sodium belongs to the alkali metal group like lithium, and its key properties—such as ionic radius, weight, and standard reduction potential—are similar to those of lithium compared to other elements. These similarities allow relatively easy application of lithium-ion battery cell structures and design methods to sodium-ion batteries, which makes them highly regarded.
Technological Challenges in Commercializing Sodium-Ion Batteries
Sodium-ion batteries have many strengths and are expected to become a key next-generation battery with continued research and development. So, where should research focus?
First, higher energy density is necessary for stable commercialization. A sodium atom is more than 3.3 times heavier than a lithium atom, which means it offers lower capacity per unit weight of electrode material. Furthermore, sodium has a higher standard reduction potential at -2.71 V, compared to lithium’s -3.04 V, leading to lower operational voltage in the same structure. These factors collectively result in a gravimetric energy density of about 140–160 Wh/kg for sodium-ion batteries, which is considerably lower than that of lithium-ion batteries.
The larger ionic radius of sodium compared to lithium has presented various challenges. It can cause structural changes during intercalation and deintercalation in cathode materials, potentially affecting battery life. It also hinders the intercalation reaction within the layered structure of graphite, a common anode material in lithium-ion batteries. For this reason, instead of graphite, researchers are investigating hard carbon, which has a wider interlayer spacing than graphite, as an anode material for sodium-ion batteries.
Improving the initial charge–discharge efficiency of hard carbon, an anode material for sodium-ion batteries, is also essential. Hard carbon in sodium-ion batteries typically shows 80–90% initial charge–discharge efficiency, which is lower than that of graphite in lithium-ion batteries. Low efficiency raises the cell’s irreversible capacity1, which can in turn reduce energy density. Accordingly, material research is underway to improve not only the capacity but also the initial charge–discharge efficiency of hard carbon.
In addition, the stability of sodium transition-metal oxides, a cathode material for sodium-ion batteries, needs to be addressed. These oxides are not highly stable in air and are highly sensitive to humidity. In humid environments, they may cause excessive loss of sodium ions from inside the cathode, undermining electrochemical performance. They may also react with moisture at the surface to form transition-metal oxides or hydroxides. These reactions can alter electrode properties and degrade battery performance, making material research on sodium transition metal oxides a key priority.
Roadmap for Sodium-Ion Battery Development by LG Energy Solution, a Pioneer in Next-Generation Batteries
LG Energy Solution has identified next-generation batteries as a key growth engine in the secondary battery sector and has devoted itself to advancing technology development. Among these, the company has distinguished itself in sodium-ion battery innovation, bringing itself to the forefront of industry attention. In March 2025, the company unveiled its sodium-ion battery roadmap at InterBattery 2025, a secondary battery conference held at COEX in Seoul.

Considering their high-power characteristics, first-generation sodium-ion batteries targeted either the replacement of lead-acid batteries or the development of 12/24V products for automotive applications and the backup power market for uninterruptible power supply (UPS) systems. LG Energy Solution is intensifying research with the goal of commercializing these first-generation sodium-ion batteries by 2027.
At the same time, the company is accelerating the development of second-generation sodium-ion batteries. For this generation, it aims to achieve an energy density of 450 Wh per liter and expand into the EV battery sector. It also seeks to ensure production efficiency and cost-effectiveness by introducing the dry electrode method to replace the conventional wet-coating method. In particular, LG Energy Solution plans to begin sodium-ion battery production proactively, with a strategy of leveraging existing lithium-ion battery production lines for manufacturing.
Sodium-ion batteries are pioneering territory in the next-generation battery industry. LG Energy Solution recognized its potential early on and has pursued a strategic development to expand into diverse applications. Looking ahead, you can expect LG Energy Solution’s sodium-ion batteries to reshape the industry landscape.
- Irreversible capacity: the amount of electric charge that cannot be discharged after charging ↩︎





