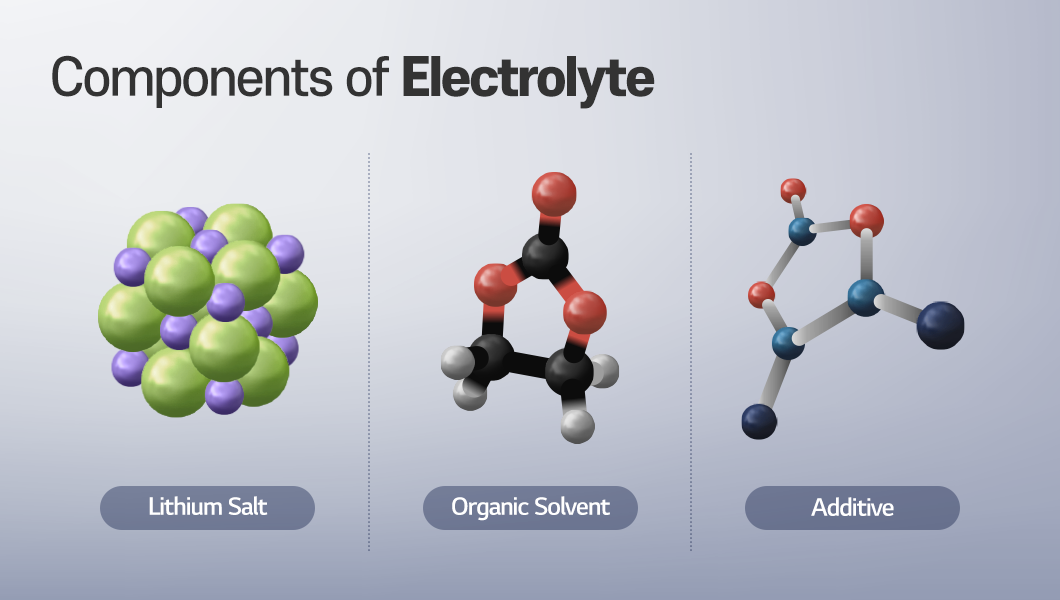
A lithium-ion battery is an energy storage device in which lithium ions travel between the cathode and anode, creating an electrochemical response during charging and discharging. In this process, the electrolyte enables the smooth transport of lithium ions. The electrolyte used in lithium-ion batteries is in liquid form to enhance ion conductivity and consists of lithium salts, organic solvents, and additives. Did you know that this electrolyte plays a critical role in determining battery performance and safety? We will take a closer look at the details by examining the roles of lithium salts, organic solvents, and additives as well as how they interact with each other.
Lithium Salts: Supplying Lithium to the Electrolyte
The “salt” in lithium salts refers to a chemical ionic compound produced by combining metal ions (cations) and non-metal ions (anions). Simply put, lithium salts have a crystalline structure where lithium ions (Li+) are bonded with anions (F–, CI–, or PF6‑). They exist in solid form and are easily soluble in water.
In lithium-ion batteries, lithium salts serve an important function by supplying lithium ions to the electrolyte. When dissolved in water or organic solvents, lithium salts undergo ionization, separating into cations and anions. The resulting lithium ions (cations) move through the electrolyte, facilitating charge1 transfer between the electrodes. This process increases the electrolyte’s ionic conductivity2, which contributes significantly to battery performance.
The characteristics of ideal lithium salts are as follows:
① High solubility in organic solvents to enable smooth transport of lithium ions within the electrolyte
② Anions that are not easily oxidized or reduced in organic solvents, and contribute to the formation of the solid electrolyte interphase (SEI)3
③ No reaction with key battery components such as electrode active materials, electrolytes, and separators, while maintaining chemical stability in various environments
Commonly used lithium salts include LiPF₆ and the increasingly popular LiFSI (F electrolyte).
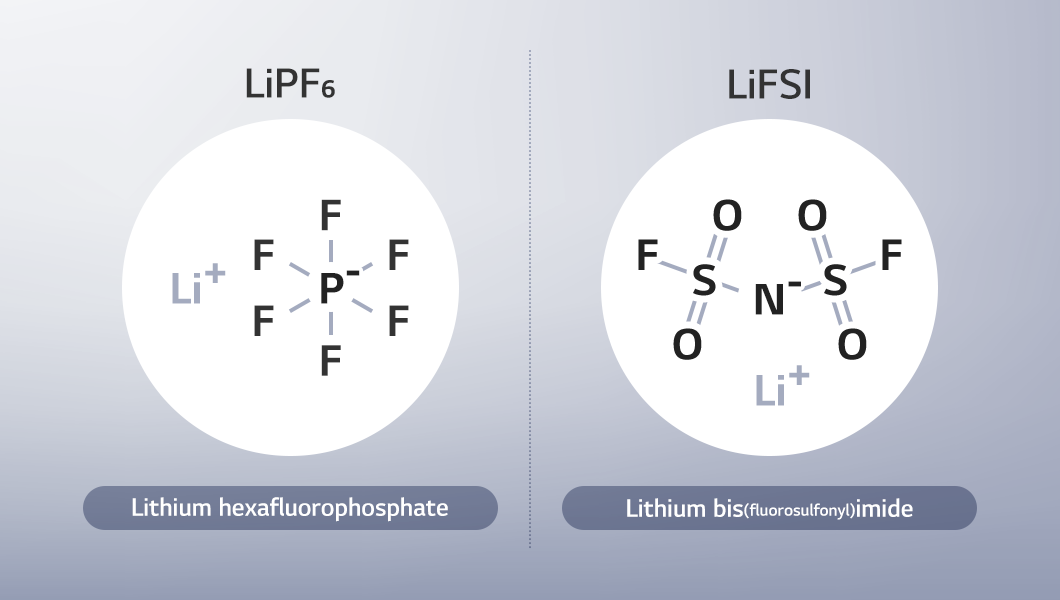
■ LiPF₆ (Lithium hexafluorophosphate): Composed of lithium ions (cations) and hexafluorophosphate (PF₆-, anions), LiPF₆ is the most widely used lithium salt due to its high ionic conductivity, low-temperature stability, and excellent solubility. However, its drawbacks such as increased thermal instability at high temperatures and strong moisture sensitivity, which can lead to the generation of hydrogen fluoride (HF)4, have prompted ongoing research into additives. LiPF₆ is primarily adopted for large-scale applications including EVs and renewable energy systems, but it is also widely applied in batteries for small electronic devices.
■ LiFSI (Lithium bis(fluorosulfonyl)imide): A lithium salt composed of lithium ions (cations) and bis(fluorosulfonyl)imide (FSI-, anions). LiFSI is considered a strong alternative to the widely used LiPF₆, as it offers higher ionic conductivity and stronger chemical stability, and remains stable in humid and high-temperature environments. It is also referred to as F electrolyte, because it contains fluorine.
LiFSI’s low electric resistance and low viscosity enable the smooth transport of lithium ions within the electrolyte. These properties boost battery output and suppress internal corrosion, contributing to extended battery life. In addition, they help prevent rapid discharge at low temperatures and maintain stability in high-voltage conditions.
In particular, LiFSI forms a stable solid electrolyte interphase (SEI). LiFSI-based lithium salts create a lithium fluoride (LiF)-rich SEI layer on the surface of lithium metal. This layer acts as an electronic insulator, limiting electrolyte decomposition and preventing dendrite5 growth. As a result, charging and discharging efficiency improves, along with battery life.
Thanks to these advantages, LiFSI is used mainly as a lithium salt, but is also applied as an additive blended with conventional electrolytes. Since high output, long life, and fast charging are essential to optimizing EV battery performance, applications and significance of LiFSI are expected to grow further.
The following lithium salts are also employed as additives to enhance electrolyte performance.
■ LiPO₂F₂ (Lithium perfluorophosphate): Helps improve battery life, shorten charging time, and support stable operation at high temperatures.
■ LiDFOP (Lithium difluorophosphate): Helps improve battery life, shorten charging time, and supports stable operation at high temperatures.
■ LiBOB (Lithium bis(oxalato)borate): Helps enhance output at room and low temperatures and increase peak power output. It also improves silicon anode lifespan and improves the performance of all-solid-state batteries.
■ LiDFOB (Lithium difluorophosphate): Demonstrates outstanding performance at low temperatures and improves battery cycling efficiency and safety by promoting stable SEI layer formation on the anode.
■ LiTFSI (Lithium bis(trifluoromethanesulfonyl)imide): Offers strong thermal safety and high ionic conductivity and maintains stability due to its low reactivity to moisture.
Organic Solvent: Supporting the Free Movement of Ions
Organic solvents dissolve lithium salts. As lithium is highly reactive with water, organic solvents are used instead. This method weakens the bond between cations and anions by dissolving lithium salts and creates an environment where the separated ions can move freely between the cathode and anode.
The characteristics of ideal organic solvents are as follows:
① High dielectric constant6 and high solubility: Effectively separates lithium ions from lithium salts.
② Low melting point: Maintains a liquid state in low-temperatures, allowing smooth lithium-ion movement.
③ High boiling point: Maintains stability by resisting evaporation at high temperatures.
④ Low viscosity: Allows ions to move faster, improving ionic conductivity.
⑤ Demonstrates high chemical stability, inhibiting degradation of the electrode structures and dissolution of the cathode and anode active materials.
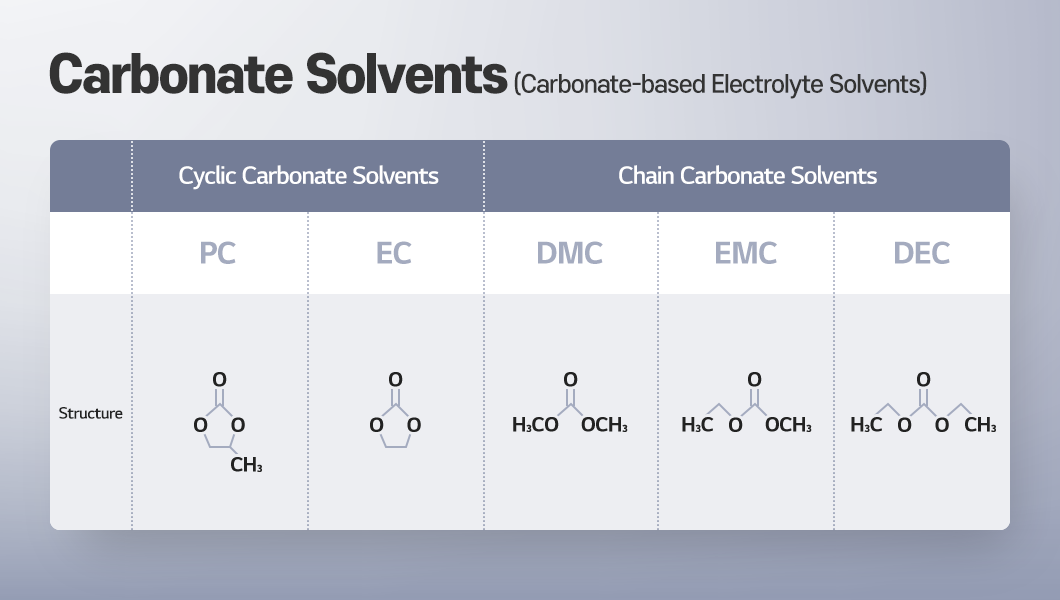
Organic solvents can be categorized into high-dielectric constant, high-viscosity cyclic carbonates and low-dielectric constant, low-viscosity chain carbonates (e.g., DEC, EMC, DMC). Cyclic carbonates can effectively dissolve lithium salts with their high dielectric constant, allowing cations and anions to separate easily. However, their high viscosity can slow down the movement of lithium ions. To address this issue, low-viscosity chain carbonates (or linear carbonates) such as DEC, EMC, or DMC are blended in.
Additives: Delivering Strong Effects with Only 5%
Additives are incorporated to support battery performance and safety across various aspects including electrolyte stability, ionic conductivity, and interfacial properties. Although they represent only 5% of the electrolyte, they account for 40% of its cost, as they play a crucial role in differentiating battery characteristics according to development targets. As a result, additive development is gaining significant attention in the industry for its high added value.
Apart from the lithium salts such as LiFSI, LiPO2F2, LiDFOP, and LiBOB, many other types of additives are also in use.
● AN (Adiponitrile) : Offers high safety through its low volatility, helps improve energy capacity and density, and extends battery life. It also exhibits exceptional solubility when combined with lithium salts.
● SN (Succinonitrile) : Provides similar effects to AN, with notable thermal stability. It also helps prevent copper dissolution.
● VC (Vinylene Carbonate) : Forms a high-quality SEI layer on the anode surface, selectively allowing lithium ions to pass while blocking electrons. This feature improves anode stability.
● FEC (Fluoro Ethylene Carbonate) : Promotes SEI layer formation, thereby accelerating lithium-ion transport, protecting the anode, and inhibiting electrolyte decomposition. This feature enhances low-temperature performance and extends battery life.
● VEC (Vinyl Ethylene Carbonate) : Improves battery life and energy density by suppressing side reactions at the electrode and maintains stability even under high voltage.
Future Research Directions
As the demand for higher-performing batteries grows, the development of next-generation electrolytes is also drawing attention. Notably, the rising interest in fast charging has driven the exploration of various new solvents. Researchers are taking various approaches, such as improving electrolyte penetration into electrode particles and enhancing lithium-ion mobility.
Electrolytes are also being studied based on the characteristics of each cathode material. Among these, for LFP cathodes, composed of lithium, phosphate, and iron, researchers are investigating additives that can further extend the LFP battery’s life and enhance stability. To this end, they are proactively exploring lithium salt-based additives.
As for electrolytes used for ternary and quaternary cathode materials, typically composed of nickel, cobalt, and manganese, research is focused on achieving stable integration with high-nickel cathode active materials and silicon-based anode active materials. This is because nickel content continues to rise in pursuit of higher energy density, and research into silicon-based anode active materials is accelerating as a potential replacement for graphite. However, high-nickel cathodes tend to increase resistance, while silicon-based anode materials can introduce instability. To address these challenges, researchers are proactively exploring additives, particularly HF scavengers and elastic SEI additives.
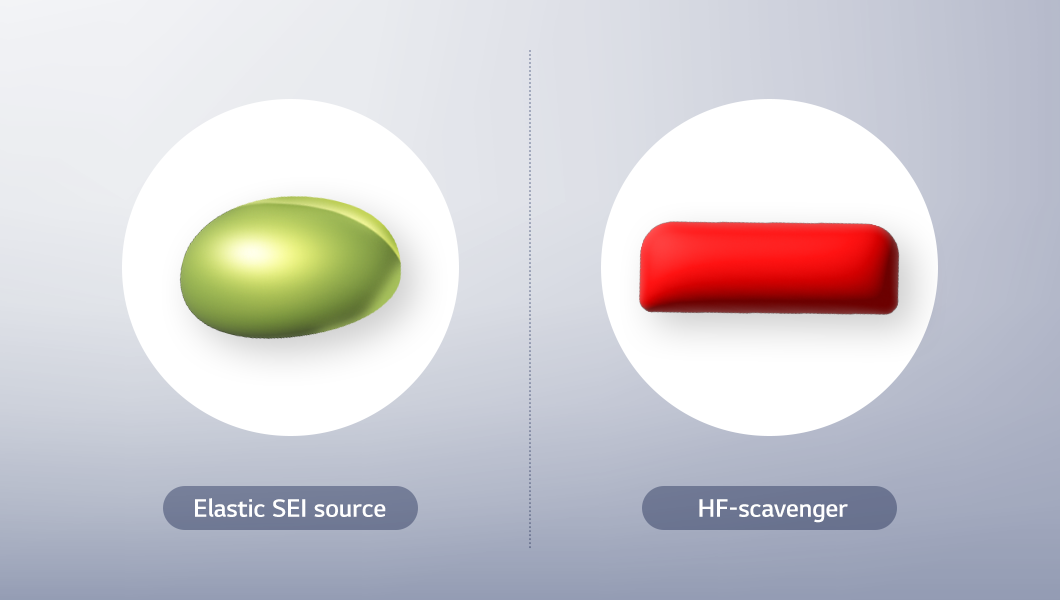
HF-scavenger neutralizes hydrofluoric acid (HF) generated through chemical reactions, thereby preventing damage to internal battery components. Meanwhile, Elastic SEI serves to stabilize the solid electrolyte interphase (SEI) layer by protecting newly formed surfaces that result from the volume expansion of silicon anodes. These types of additives help suppress resistance even at temperatures around 60℃ and, over time, demonstrate lower resistance compared to conventional electrolytes—ultimately contributing to enhanced battery safety and performance.
As the demand for batteries continues to grow, the development of electrolyte technologies, along with research into their components such as lithium salts, organic solvents, and additives, is becoming increasingly important. According to a global market research agency, the global demand for electrolytes is expected to grow at an average annual rate of 11% over the next 11 years, reaching 4.46 million tons by 2035. In addition, the global electrolyte additive market size was valued at USD 1.43 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 16.4%, reaching USD 5.56 billion by 2032.
As the improvement of electrolyte performance emerges as a key future challenge in battery technology development, such advancements are expected to further expand the potential for application across various fields.
- Charge: The quantity of static electricity present on an object. It serves as the source of all electric phenomena. Charges are divided into positive and negative charges, and their movement constitutes electric current. ↩︎
- Ionic conductivity: An indicator of how ions move through an electrolyte to transfer charge (generate electricity). ↩︎
- Solid Electrolyte Interphase (SEI): A layer formed on the anode surface during the battery’s initial charging. ↩︎
- Hydrogen Fluoride (HF): A highly toxic and corrosive gas that is readily soluble in water. ↩︎
- Dendrite: Lithium deposits that form on the anode surface during charge/discharge cycles, potentially reducing battery life or damage the separator. ↩︎
- Dielectric constant: The extent to which a material responds to an electric field. ↩︎





