Last time, we covered the types of lithium and their differences as lithium is receiving attention as a cathode material for EV batteries. This time, we will learn why lithium hydroxide is used for making high-capacity batteries.

Q. Why is lithium hydroxide necessary for making lithium-ion batteries?

Lithium-ion batteries are composed of four key components: cathode, anode, electrolyte, and separator. And they generate electricity through chemical reactions in which lithium ions move between the cathode and anode.
Lithium has a strong tendency to lose electrons to become positive ions, making it suitable as a cathode material, and a cathode consists of a compound containing lithium.
Since a cathode determines the battery’s capacity and average voltage, it plays a crucial role in battery performance. The higher the proportion of lithium in a cathode, the greater the capacity of the battery. Also, as the battery voltage is determined by the potential difference between two points, the structure of the cathode affects the electric potential and has a great impact on voltage.
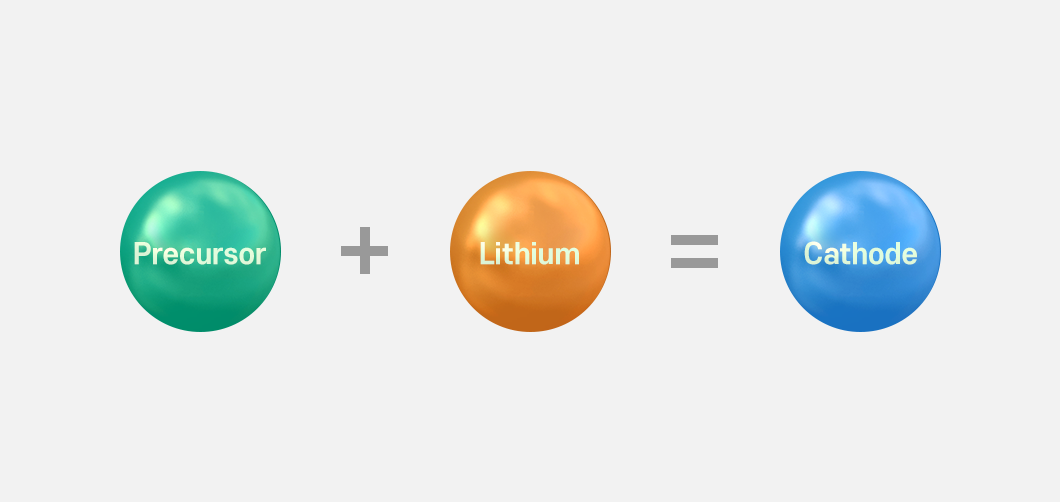
So, how are the cathodes made? To make cathodes, the “precursor,” a compound produced by mixing raw materials such as nickel and cobalt, is necessary. A precursor is a term used to refer to a material at just before becoming the final material A in producing the material A through a chemical process. In battery manufacturing, a precursor is a raw material for cathode, which can include nickel, cobalt, and manganese. A precursor compound is made by mixing these raw materials, and lithium is added to it. Finally, a cathode is made when additives such as conductive additives and binders are put into it.
Q. Why is lithium hydroxide used for high-capacity batteries?
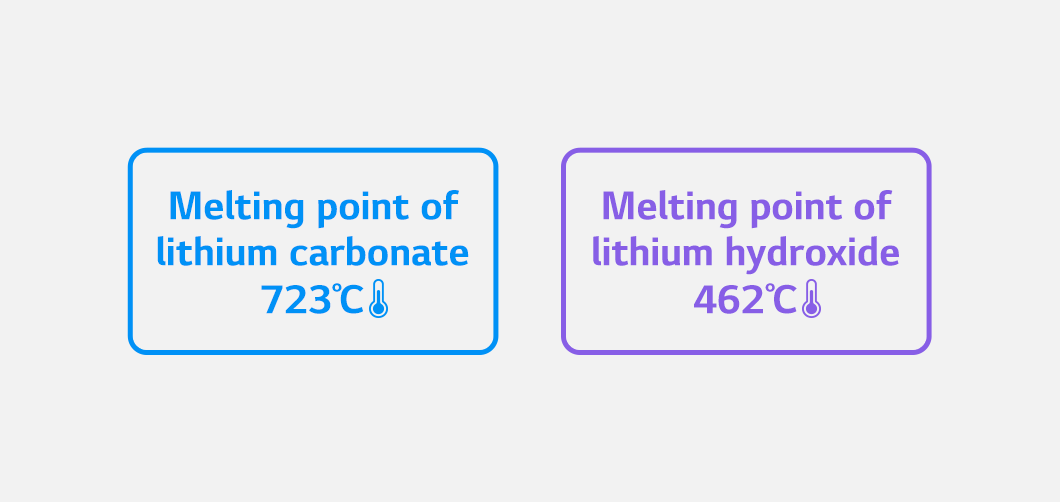
Lithium compounds used in manufacturing lithium-ion batteries are largely divided into lithium carbonate and lithium hydroxide.
Lithium carbonate is mainly used for LFP batteries that use iron phosphate as a cathode material or batteries for home appliances and IT devices, while lithium hydroxide is used for high-capacity batteries such as high-nickel batteries.
Lithium hydroxide is employed for manufacturing high-capacity batteries because it is easily synthesized with nickel. Nickel does not synthesize well with lithium at high temperatures. Lithium hydroxide has a lower melting point than lithium carbonate and is therefore preferred.
Q. What is the prospect of the lithium hydroxide market?
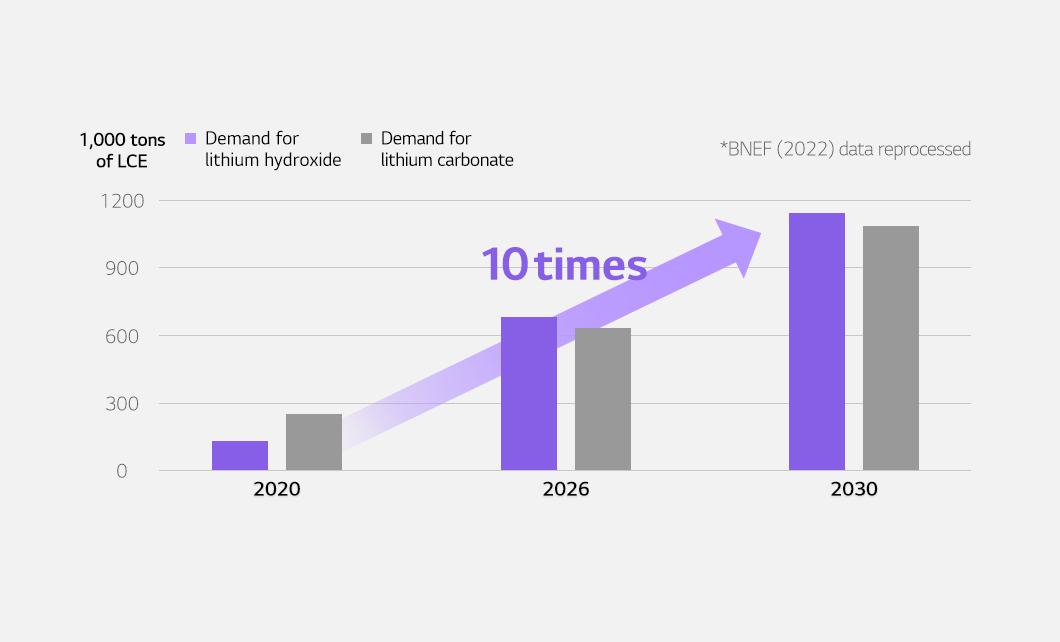
According to BloombergNEF (2022) data, the demand for lithium hydroxide is forecast to surpass that of lithium carbonate in 2026. The rise is expected to be mainly driven by the surge in demand for high-performance batteries as well as high-nickel batteries for EVs. The demand for lithium hydroxide is expected to skyrocket more than 10 times in 2030 compared to 2020.
As a result, competition among relevant companies to secure lithium hydroxide is expected to intensify. LG Energy Solution is striving to establish a stable lithium supply network to preemptively respond to the trend by signing lithium hydroxide supply agreements with leading global companies.





