
The world is striving for a massive shift from fossil fuels to renewable energy. To address the climate crisis, various innovative technologies are being developed. LG Energy Solution is also speeding up the development of next-generation batteries, with the mission of the times serving as a driving force. A lecture, “Beyond Li-Ion Battery: The Better, The Safer,” held on March 16th at LG Science Park in Magok, Gangseo-gu, Seoul, attracted many R&D talents with master’s and doctoral degrees in science and engineering who are interested in next-generation batteries. What is LG Energy Solution’s vision for the next-generation battery? Let’s find it out by listening to Seung-hun Han from Lithium Sulfur Battery 2 PJT Team at Next Generation Battery Development Center
Envisioning Next-Generation Batteries

What is a next-generation battery? “Batteries that aren’t in use right now but that people want to use in the future.” Many people may identify with this definition. A more sentimental definition might be “batteries capable of dominating the era.” Lithium-ion batteries are thriving in the modern era. So, if it is a next-generation battery, it must be able to replace a significant portion or the majority of lithium-ion batteries.
When I was a kid, I used to play with minicars a lot. Nickel-cadmium (Ni-Cd) batteries, also known as NiCad batteries, were widely used then. What kind of battery would a kid who likes to play with minicars want? First, I would want a battery that lasts longer because I don’t want to replace it too often. Second, whether a minicar wins or loses is determined by the motor and battery output, so I want the output to be good. Third, I want it to be safe so that no fires or explosions occur. Fourth, I hope the price is reasonable enough for a child to afford it.
In summary, the following are the requirements for the battery I want.
First, more energy (Wh/kg, Wh/L). Second, higher output (kW/kg, kW/L). Third, increased security (no fire). Fourth, lower price ($/kWh). What are the next-generation battery candidates that meet these criteria?
To begin, there is a lot of interest in all-solid-state batteries. Then there are lithium-sulfur and lithium metal batteries, which use lithium metal as an anode. Also included in the candidate group are lithium-air batteries that use gas as an energy source.
“We’ll find a way. We always have.” This is a quote from the poster for the film, Interstellar, and it also describes my approach to researching next-generation batteries. Because next-generation batteries are not currently in use and are difficult to use, the term “next-generation” is used. However, I believe that one day we will find a way, and if we do, I will be the one to find it.
Returning to the Origins of Lithium-Ion Batteries

Let us begin with the past before moving on to the future. Do you know the three professors awarded the 2019 Nobel Prize in Chemistry for their contributions to commercializing lithium-ion batteries? Three distinguished professors: John Goodenough, Stanley Whittingham, and Akira Yoshino. While the work of Professors John Goodenough and Akira Yoshino on cathode and anode materials are well-known, Professor Stanley Whittingham’s contributions may be less famous. So I’m going to look into this person’s past.
Let’s go back to the 1970s when lithium-ion batteries were just getting started. Professor Stanley Whittingham demonstrated a prototype lithium rechargeable battery. The anode material used then was not graphite, as is commonly used today, but lithium metal, which can explode when exposed to water. The cathode is comparable to the present in this case, but it is no longer in use. Why can’t we use a rechargeable battery like this now? It is because of a possible short circuit. Whiskers and dendrites, both sharp dendrites, can cause a short circuit if they grow through the separator and touch the anode and cathode.
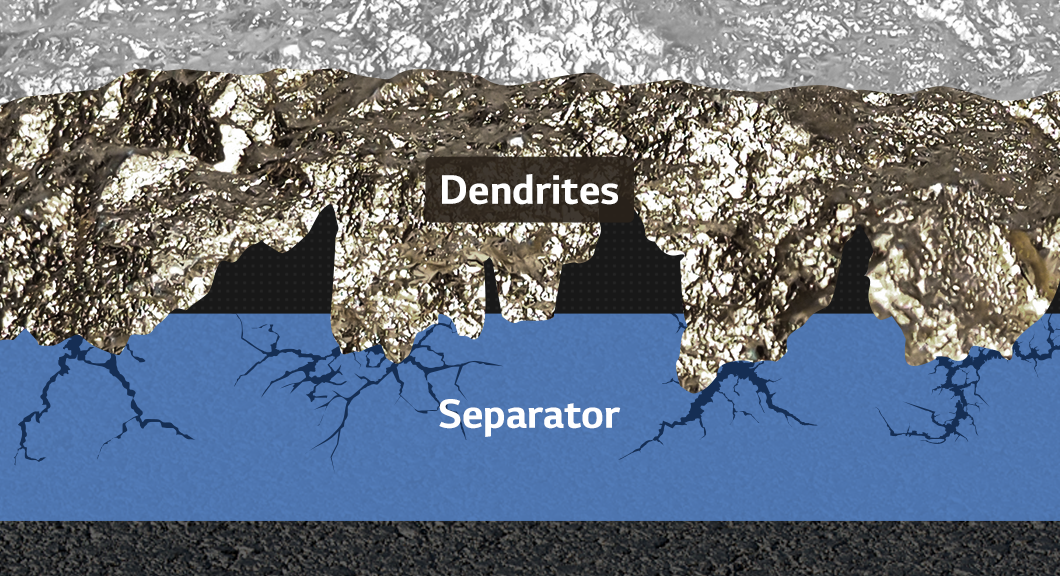
The lithium metal and short circuit problems are the challenges of the next-generation battery we are developing. They are the ones that Professor Whittingham has previously identified. In other words, if we can build a working prototype lithium-ion battery, we can build a next-generation battery.
There Is No Place Like Home, Right?

Now let’s go back to the lithium metal battery. The adage “There is no place like home (or host)” also applies here. Lithium ions in a lithium-ion battery are safely housed in a structure known as a host material. The graphite anode material is like a cozy home for lithium ions. It is quite stable since the ions only need to enter it.
On the other hand, lithium metal batteries use lithium metal as an anode material. It no longer has a home made with graphite. When the anode material is replaced with lithium metal, lithium ions are reduced and become lithium metal; when they are oxidized, they become lithium ions. When lithium ions exit lithium metal during a charging and discharging cycle, they no longer have a place to return to. When the battery is charged, and the ions want to return to the lithium metal anode, they must construct their own home. As a result, lithium metal can be considered a temporary daily residence for lithium ions.
So, has the past short circuit problem been resolved? It has greatly improved. Ironically, the development of lithium-ion batteries greatly aided in the usability of lithium-metal batteries. First, a plethora of materials and technologies emerged. We developed new electrolytes and electrolyte additives for lithium-ion batteries that can stabilize lithium metal. Although lithium metal batteries do not have a host, academia has been researching lithium metal protective layers so that the ions can build a better home when building one. There has also been researching on separators that make it difficult for dendrites to penetrate.
Returning to the Dry Cell

Now, let’s talk about an all-solid-state battery. Since every component of an all-solid-state battery is solid, they can be considered dry cells. The idea is straightforward. Only the electrolyte, out of the four major materials in a lithium-ion battery (anode, cathode, separator, and electrolyte), needs to be changed to a solid electrolyte. However, when attempting to do so, the separator becomes a problem. Wet separators are required for high-capacity batteries, but solids cannot be immersed in water. As a result, the separator must also be solid. Because the solid is heavier than the liquid, achieving more energy and higher output, in this case, is difficult.
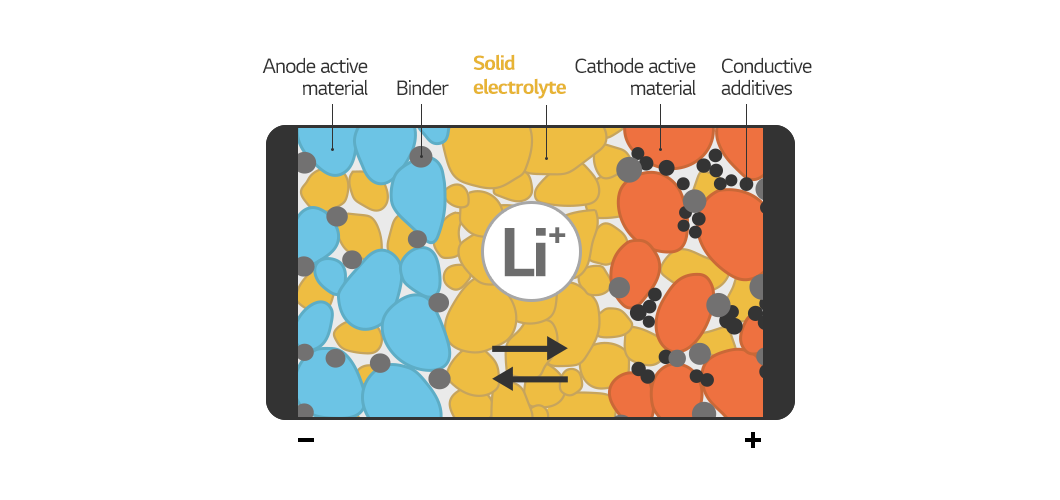
As a result, we are conducting research to develop all-solid-state batteries with greater energy and output than conventional lithium-ion batteries by changing the anode material to metal or making it anode free. In particular, we are constantly researching technologies to improve the ionic conductivity of solid electrolytes and make solid membranes thinner and stronger to solve the problem of solids not spreading well.
What Would the All-Solid-State Battery From LG Energy Solution Look Like?

Let’s take a look at the five components of the next-generation all-solid-state battery that LG Energy Solutions is aiming for. First, we are investigating “no anode” batteries, which do not have an active anode material. Second, there is no separator. It aims to create a “no-separator” battery that operates normally with a solid electrolyte film. Third, there should be “no short circuit” concerns. The battery should be sufficiently movable and have no safety concerns, even when internal and external deformation occurs. Fourth, there is no leakage when the battery is damaged because it contains “no liquid.” The fifth is “non-flammable,” which, even when applied directly to a fire, does not start a fire.
Future With Various Next-Generation Batteries

Let’s look for something lighter because metal is heavy. When a metal is used as the anode, the cell gets heavier. Cathode materials are typically composed of nickel, manganese, and cobalt metals, but these can be replaced with sulfur. This allows you to completely avoid the host material. In other words, there wouldn’t be any material in the battery that could store lithium ions. Instead, a chemical reaction involving sulfur produces energy. Lithium-sulfur batteries began as primary batteries. It became possible to function as a rechargeable battery in the first wave with the introduction of lithium nitrate (LiNO3). Now, as a new market emerges in the second and third waves, it appears that lithium-sulfur batteries will evolve once again.
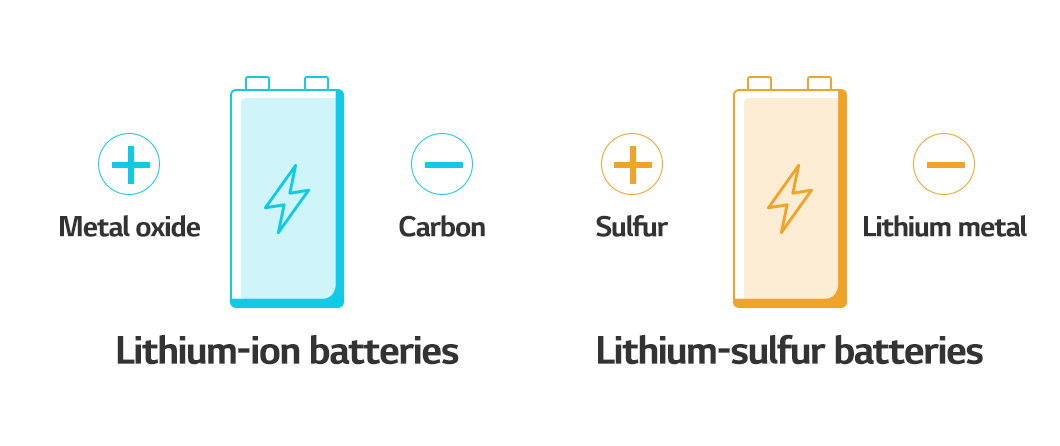
So, which of the next-generation batteries will we see first? It is anticipated that we will first encounter the lightest lithium-sulfur battery. The first all-solid-state battery is likely to be a polymer all-solid-state battery. The lithium metal batteries will have the highest energy density per volume. Finally, all-solid-state batteries based on sulfide will be the safest rechargeable batteries with a relatively high energy density per volume.





