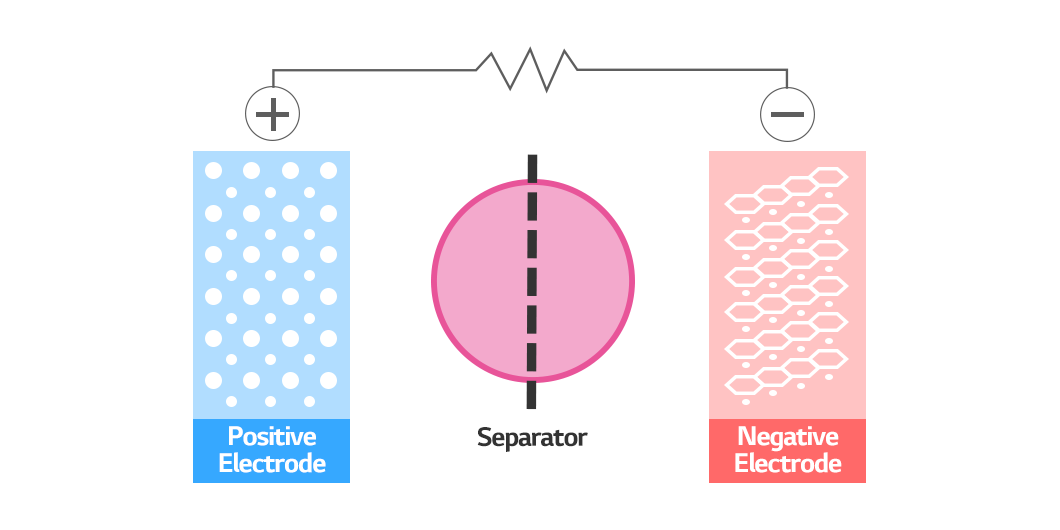
The cathode and anode determine the power of a battery among many elements of a rechargeable battery. These two electrodes should not touch each other, lest they cause a short circuit. This is where a separator comes in. The separator prevents the cathode and anode from touching each other for safety. Today, let’s take a closer look at the lithium-ion battery separator (LiBS), which is crucial when ensuring battery safety.
What is a separator?
A lithium-ion battery generates electricity through a chemical reaction produced when a lithium-ion travels between anode and cathode. A separator serves as a wall separating the anode and the cathode, as well as a passage facilitating the movement of lithium ions. This is why the separator needs to be a thin membrane made of an insulating material and have micro-pores simultaneously.
Three prerequisites of a good separator
A separator needs to play the roles of both a wall between electrodes and a passage for lithium ions. What would make a separator effective?
First, it must possess high thermal stability and electrical insulation properties. Thermal stability is critical since the direct contact of the cathode and anode may generate heat energy instead of electricity, causing a fire. In addition, a separator should be able to prevent the movement of lithium ions by blocking pores when the temperature rises beyond a certain threshold to prevent an internal short circuit. Electrical insulation is vital since the separator shouldn’t react with other ions inside the battery, including lithium ions.
Second, the pores should be consistent in size and large in number. To recap, the chemical reaction of lithium generates electricity. Lithium ions travel between the cathode and anode, causing the current to flow. As a result, the movement of lithium ions would be smoother when the pores, the passageways for lithium ions, are consistent in size and large in number.
Third, it must be thin and strong. The thinner the separator, the more material can be added to the anode and cathode, improving battery performance. However, the separator should possess sufficient physical strength to withstand external physical shock and be able to perform its role.
Dry separator vs. Wet separator
There are limited polymer materials for separators, most of which are polyethylene (PE) and polypropylene (PP). Separators are generally classified into wet or dry types depending on how the pores are created.
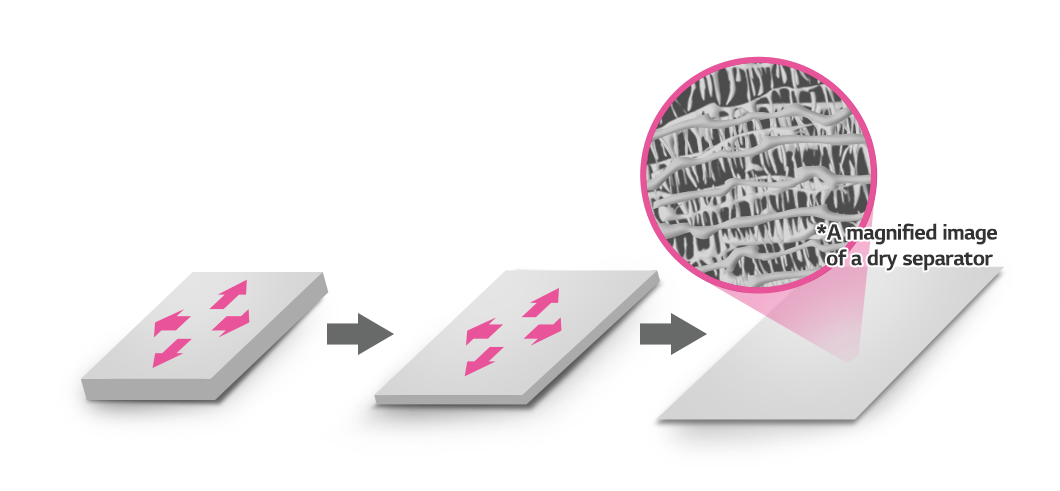
Dry separators create pores by pulling PE and PP membranes in one direction. The process is simple and suitable for mass production. However, keeping the pore size consistent and the membrane thin is not easy. As a result, dry separators are applied to areas that do not require high energy density, such as ESS (Energy Storage System) and electric city buses.
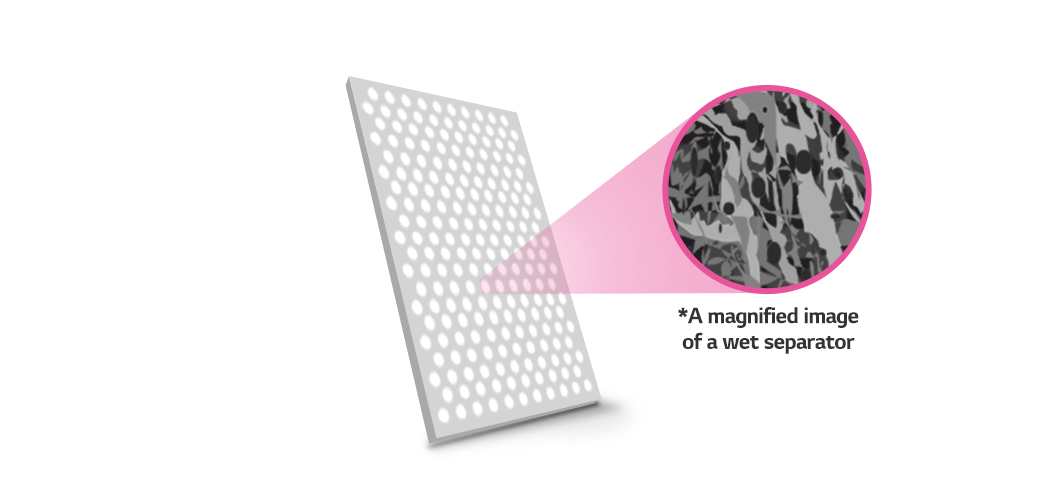
On the other hand, wet separators are made flat by extruding a mixture of PE, PP, and paraffin oil at a high temperature. The pores will appear when the extruded PE and PP sheet slowly hardens and the oil filling the gaps is extracted. Unlike dry separators, which are stretched in only one direction, wet separators have uniform pores since they are stretched in both length and width. Undoubtedly, manufacturing wet separators is more complicated than that of dry separators, requiring more materials and raising the unit cost. However, a wet separator boasts high energy density thanks to its thinness. As a result, wet separators are widely used in small battery applications such as mobile phones and laptops or high-capacity and high-output battery applications such as electric vehicle batteries.
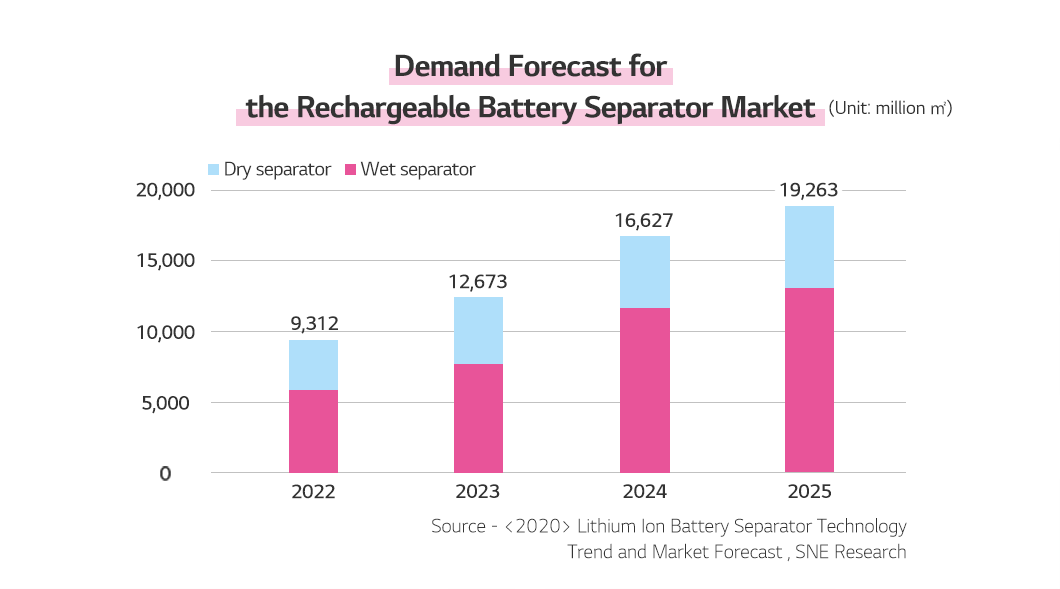
According to SNE Research’s Lithium-Ion Battery Separator Technology Trend and Market Forecast, the market demand for rechargeable battery separators is expected to grow at a CAGR of 38% until 2025. It also pointed out that the demand for wet separators would grow faster than for dry separators due to the growing battery trend of miniaturization, weight reduction, and higher capacity.
The future of separators
The global separator market demand is expected to be driven exponentially by the growth of the electric vehicle battery market. One of the hurdles that electric vehicles must clear is the driving range. One way to improve this is to increase the range by enhancing the energy density through higher cathode material content with a thinner separator. However, one caveat is that a thinner separator increases the risk of an accident as the cathode and anode could come into contact more easily. This is why many manufacturers are striving to develop thin and strong separators. As many manufacturers at home and abroad are breeding separator technologies that aim to significantly advance safety and performance, we hope to see a new generation of separators that would usher in the era of electric vehicles becoming mainstream.





